Pharmaceutical composition and applications thereof
A technology of composition and use, applied in the preparation of medicines, in the field of interleukin-6 modulators, can solve the problems of difficult acquisition and unstable properties, and achieve the effects of stable storage, convenient storage, significant application prospects and economic value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] The preparation of embodiment 1 composition of the present invention
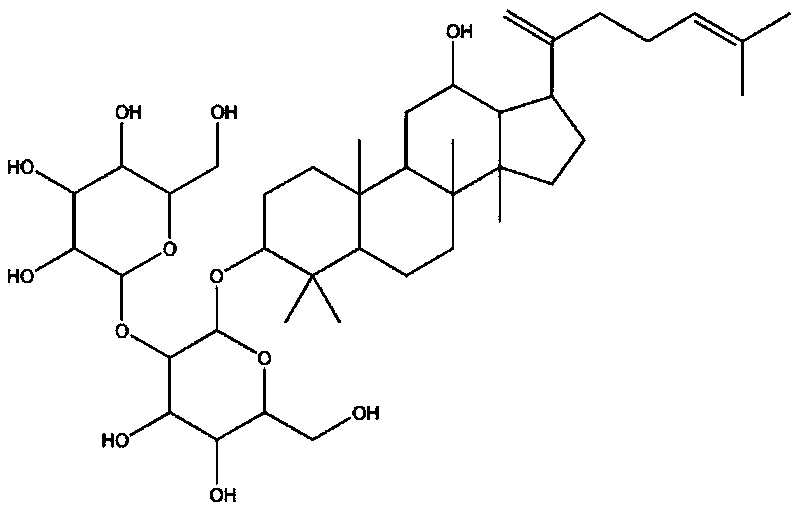
[0022] Ginsenoside Rk1, ginsenoside Rg5, and ginsenoside CK were purchased from Xi'an Baile Biotechnology Development Co., Ltd., Chengdu Master Biotechnology Co., Ltd. and extracted from red ginseng, and their purity met the medicinal standard. The composition of the present invention is prepared according to the following specific gravity:
[0023]
Embodiment 2
[0024] The safety evaluation of embodiment 2 compositions of the present invention
[0025] The study of non-clinical safety evaluation of the composition of the present invention is as follows. In the following experiments, the solution of the composition of the present invention prepared in Example 1 was added to the cell culture medium.
[0026] 1. Oral acute toxicity test in mice
[0027] Under the conditions of maximum dosage concentration and maximum dosage volume, mice were orally administered the composition prepared in Example 1, and observed continuously for 14 days to obtain the maximum tolerated dose of the composition of the present invention.
[0028] 2. Beagle dog oral acute toxicity test
[0029] Under the conditions of maximum dosage concentration and maximum dosage volume, the composition prepared in Example 1 was orally administered to Beagle dogs by gavage, so as to obtain the maximum tolerated dose of the composition of the present invention.
[0030] 3...
Embodiment 3
[0038] Embodiment 3 Pharmacodynamics
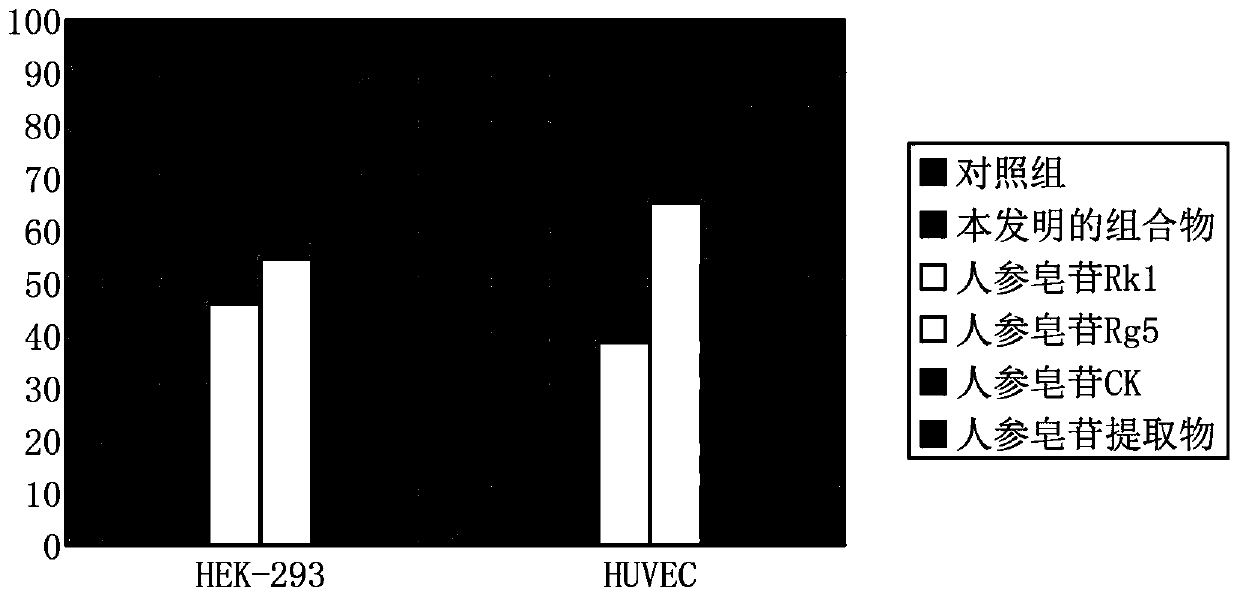
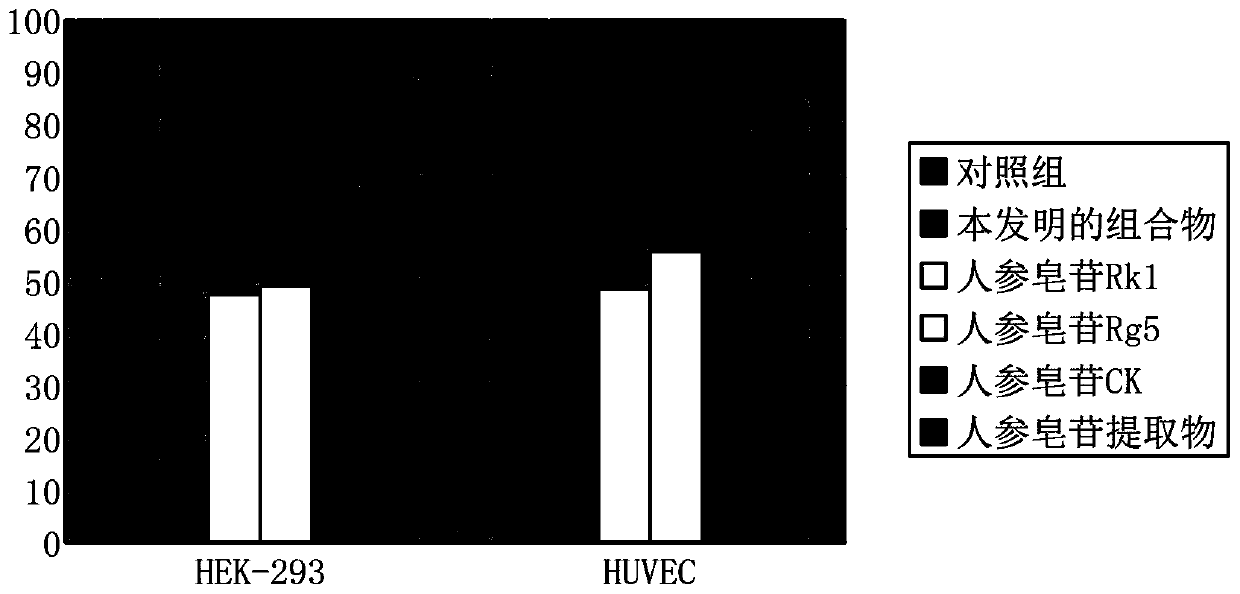
[0039] 1. The composition of the present invention inhibits the synthesis of interleukin-6 in cells
[0040] Materials: human umbilical vein endothelial cells HUVEC, human embryonic kidney epithelial cells HEK-293, and the formulated composition of the present invention. By accurately weighing 1.5 mg of the composition prepared in Example 1, and dissolving it in 200 μL of 75% ethanol solution to a final concentration of 7.5 mg / ml, the ethanol solution of the composition of Example 1 of the present invention was obtained.
[0041] By accurately weighing 1.5 mg of ginsenoside Rk1, ginsenoside Rg5, ginsenoside CK and total ginsenosides extract, and dissolving it in 200 μl of 75% ethanol solution to a final concentration of 7.5 mg / mL, the ethanol content of each of the above saponins was obtained. solution.
[0042] The control group used 200 μl 75% ethanol solution.
[0043] Methods: Human umbilical vein endothelial cells HUVEC and human ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com