Pharmaceutical applications for (s)-norketamine and salts thereof
A technology for methylketamine and medicine, which is applied in the directions of medicine combination, medical preparation containing active ingredients, medicine formula, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
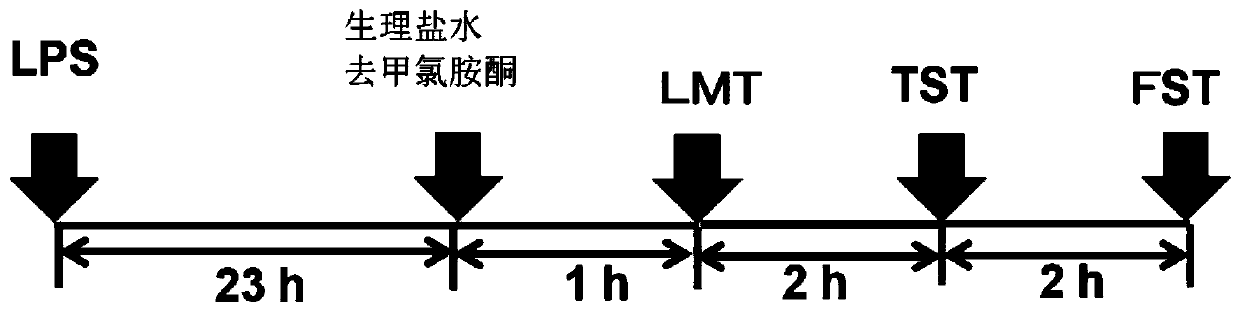
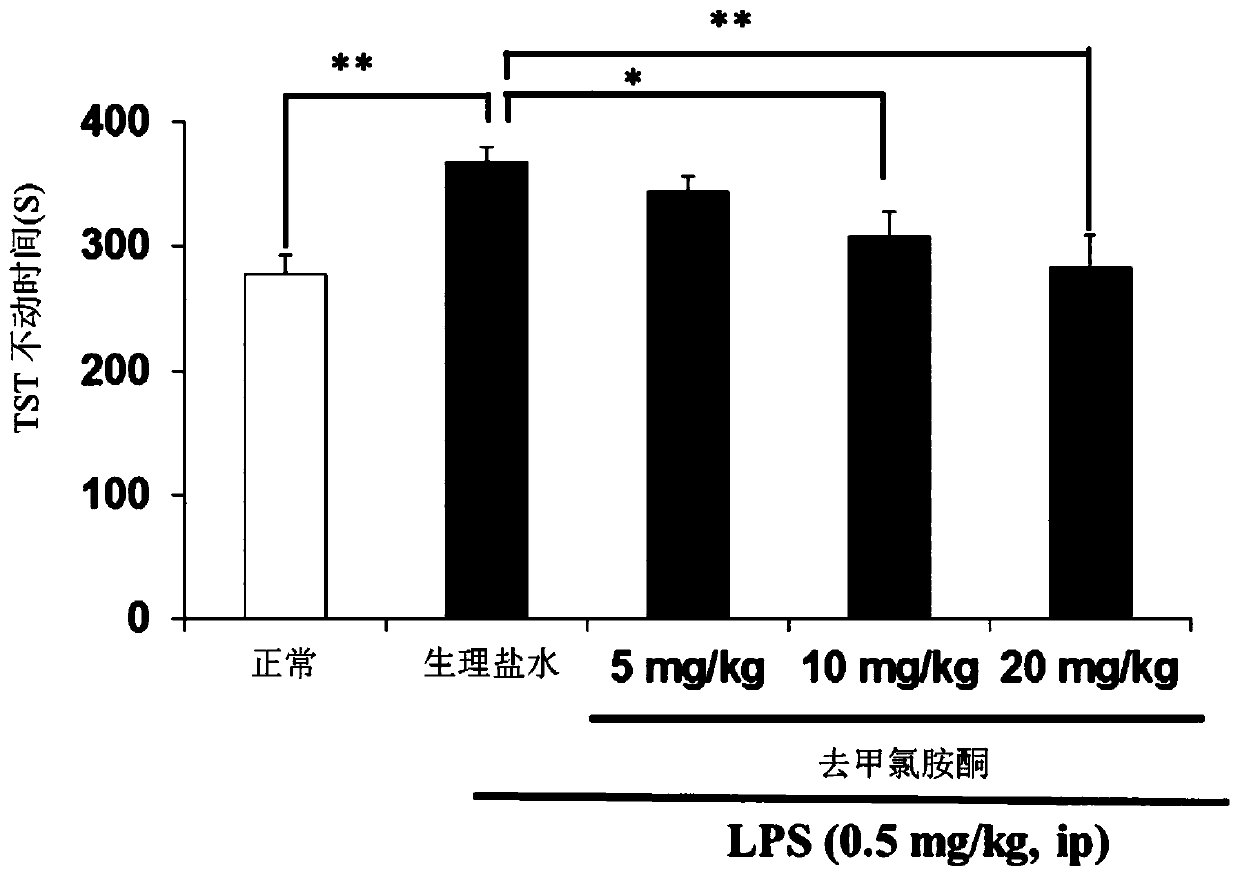
[0194] Using an inflammatory animal model of depression (non-patent literature 14-16), the antidepressant effect of norketamine, the main metabolite of ketamine, on the depression-like behavior of the model animals was investigated.
[0195] 1. Materials and Methods
[0196] Norketamine hydrochloride was purchased from Tocris Bioscience (Bristol, UK). Physiological saline was used as a negative control of the drug.
[0197] An inflammatory animal model of depression was created (constructed) by administering lipopolysaccharide (hereinafter abbreviated as LPS) to mice during adulthood. Depression-like behavior was observed in mice given LPS, suggesting that this mouse could serve as a new animal model of depression. The model mice were made by the inventors of the present application and their assistants, and were reported in many papers (Non-Patent Documents 14-16). This model mouse was used as an indicator in the screening of antidepressants in any one of the tail suspensi...
Embodiment 2
[0205] The antidepressant effect of norketamine was compared with that of ketamine. Specifically, using an inflammatory animal model of depression (Non-Patent Documents 14-16), the antidepressant effects of ketamine and norketamine on the depression-like behavior of the model animals were investigated.
[0206] 1. Materials and Methods
[0207] Norketamine hydrochloride was purchased from Tocris Bioscience (Bristol, UK). Ketamine hydrochloride (Ketalar (registered trademark)) was purchased from Daiichi Sankyo Co., Ltd. (Tokyo, Japan). Physiological saline was used as a negative control of the drug.
[0208] An inflammatory animal model of depression was prepared by administering lipopolysaccharide (hereinafter abbreviated as LPS) to the adult mice in the same manner as in Example 1.
[0209] The investigation of the antidepressant effects of ketamine and norketamine was carried out by the behavioral tests of LMT, TST and FST by the same method as that described in Example 1...
Embodiment 3
[0215] A comparative study of the side effects of ketamine and norketamine was carried out by the exercise hyperactivity test and the prepulse suppression test which are evaluation systems for side effects.
[0216] 1. Materials and Methods
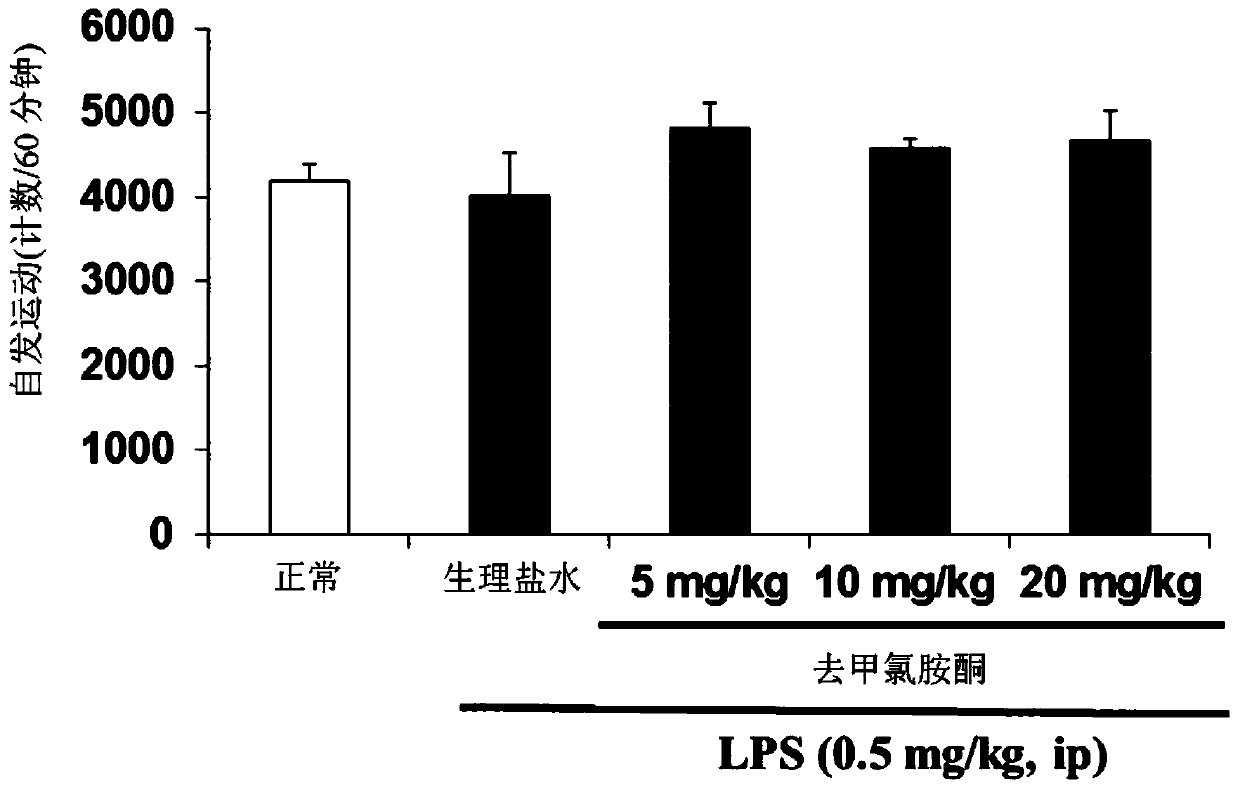
[0217] The effects of ketamine and norketamine on the amount of exercise in mice were tested using SCANET MV-40 (MELQUEST Ltd., Toyama, Japan). Specifically, the measurement was performed for a total of 180 minutes from 60 minutes before the administration to 120 minutes after the administration, and the amount of exercise per 10 minutes was calculated. Statistical analysis of the exercise amount results was performed by performing repeated one-way analysis of variance (Repeated one-way ANOVA) followed by least significant difference test (LSD test). Data are presented as mean ± standard deviation (n = 7 or 8 mice / group). The significant difference compared with the group administered with normal saline is represented by **p<0.05, ***p<...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com