Application of combination of gabapentin, neurotropin and sinomenine hydrochloride to preparation of medicine for treating PHN (postherpetic neuralgia)
A technology of neurotropin and sinomenine hydrochloride, which is applied in the field of application of the combination of gabapentin, neurotropin and sinomenine hydrochloride in the preparation of medicines for treating PHN, can solve the problem of large side effects, protracted disease, Patient compliance is not good and other problems, to achieve the effect of high safety, good clinical efficacy and less adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0023] The present invention will be further illustrated below in conjunction with the examples, but the invention is not limited to the specific examples.
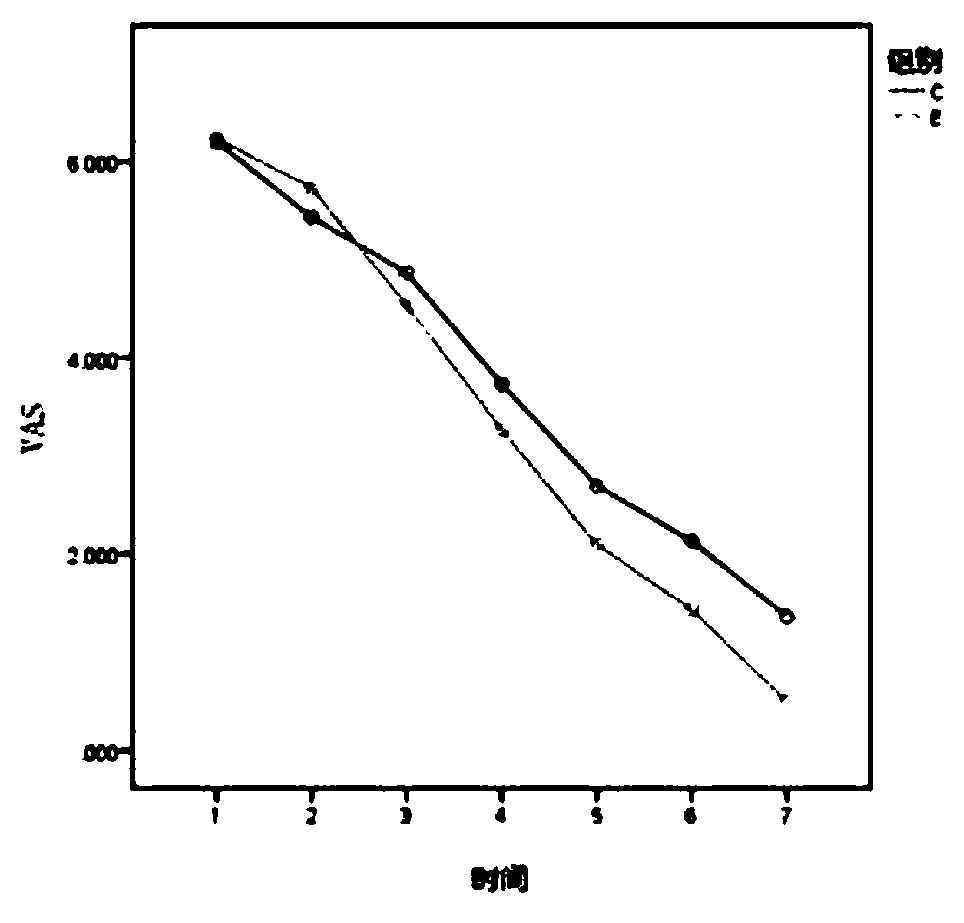
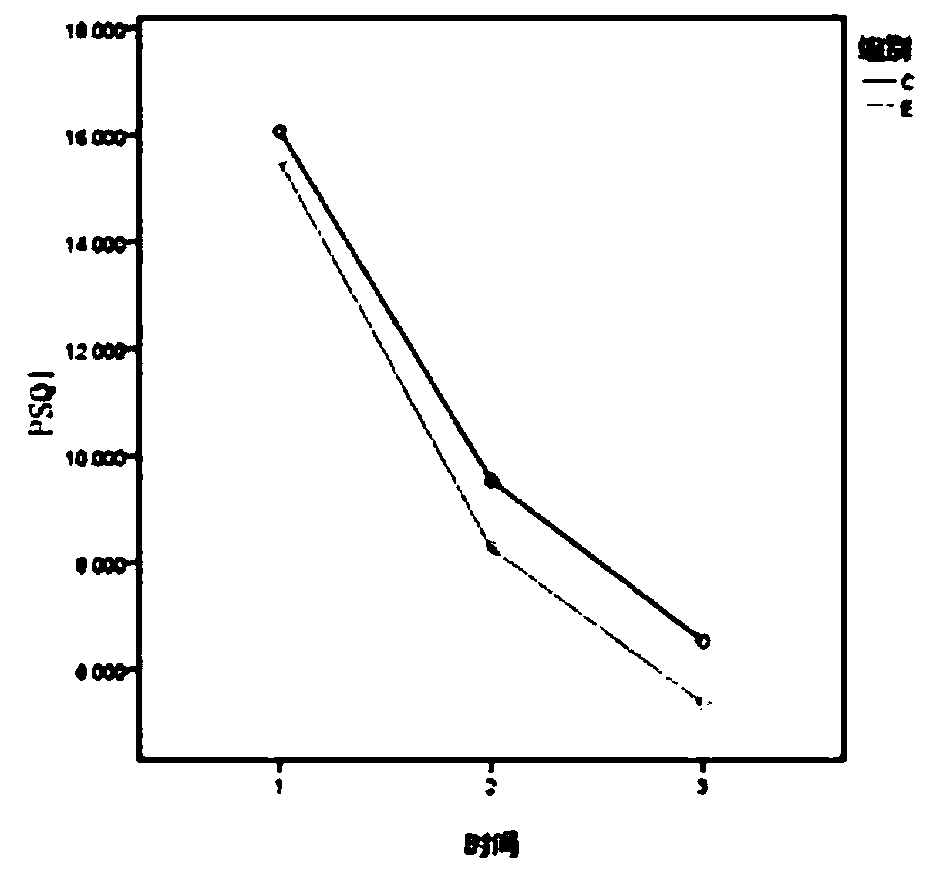
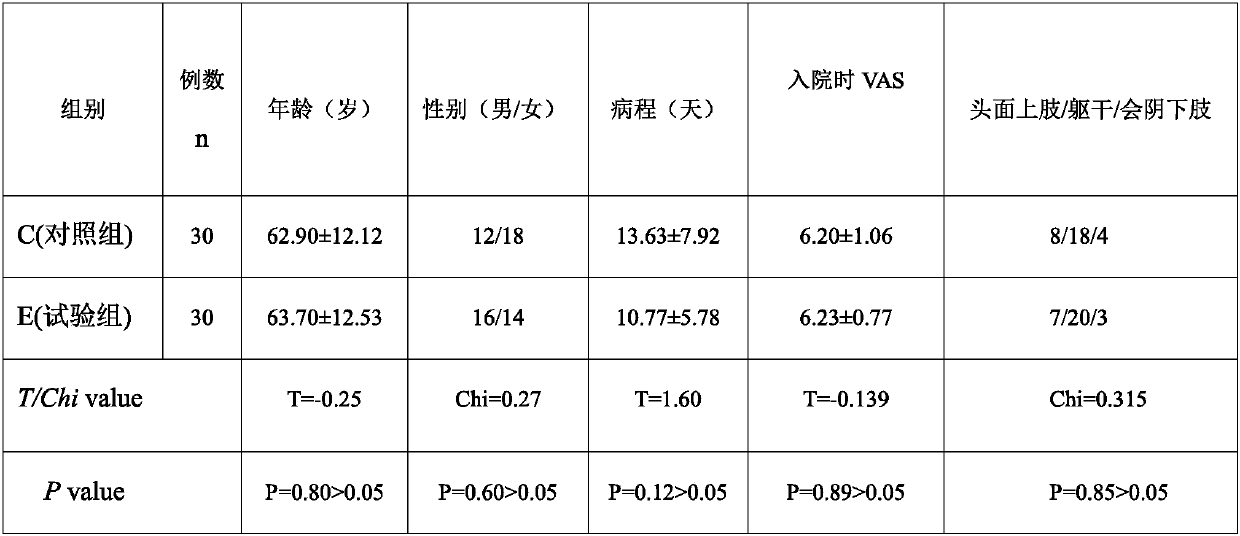
[0024] From December 2017 to December 2018, 60 patients with subacute PHN who were diagnosed in a certain department of a certain hospital were divided into the control group and the experimental group according to the random number table method.
[0025] (1) Inclusion criteria: ① meet the diagnostic criteria of subacute PHN of the International Association for the Study of Pain; ② course of disease 1-3 months; ③ age over 50 years; ④ VAS ≥ 5 points; ⑤ no nausea, vomiting, dizziness, constipation, urinary retention ⑥ Have not taken other analgesic drugs that act on the central and peripheral areas 2 weeks before the screening period.
[0026] (2) Exclusion criteria: ①painful diseases with unclear diagnosis; ②those with a history of asthma and allergy to Zhengqing Fengtongning, allergy to any component in gabapentin, patien...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com