Human antibody sample molecule TEM8-Fc based on tumour endocytosis sign 8 and its application in tumour treatment
A technology of endothelial cells and human antibodies, applied in the direction of anti-tumor drugs, medical preparations containing active ingredients, cells modified by introducing foreign genetic materials, etc., to achieve wide indications, small side effects, and large market effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1. SDS-PAGE analysis of gene recombination products
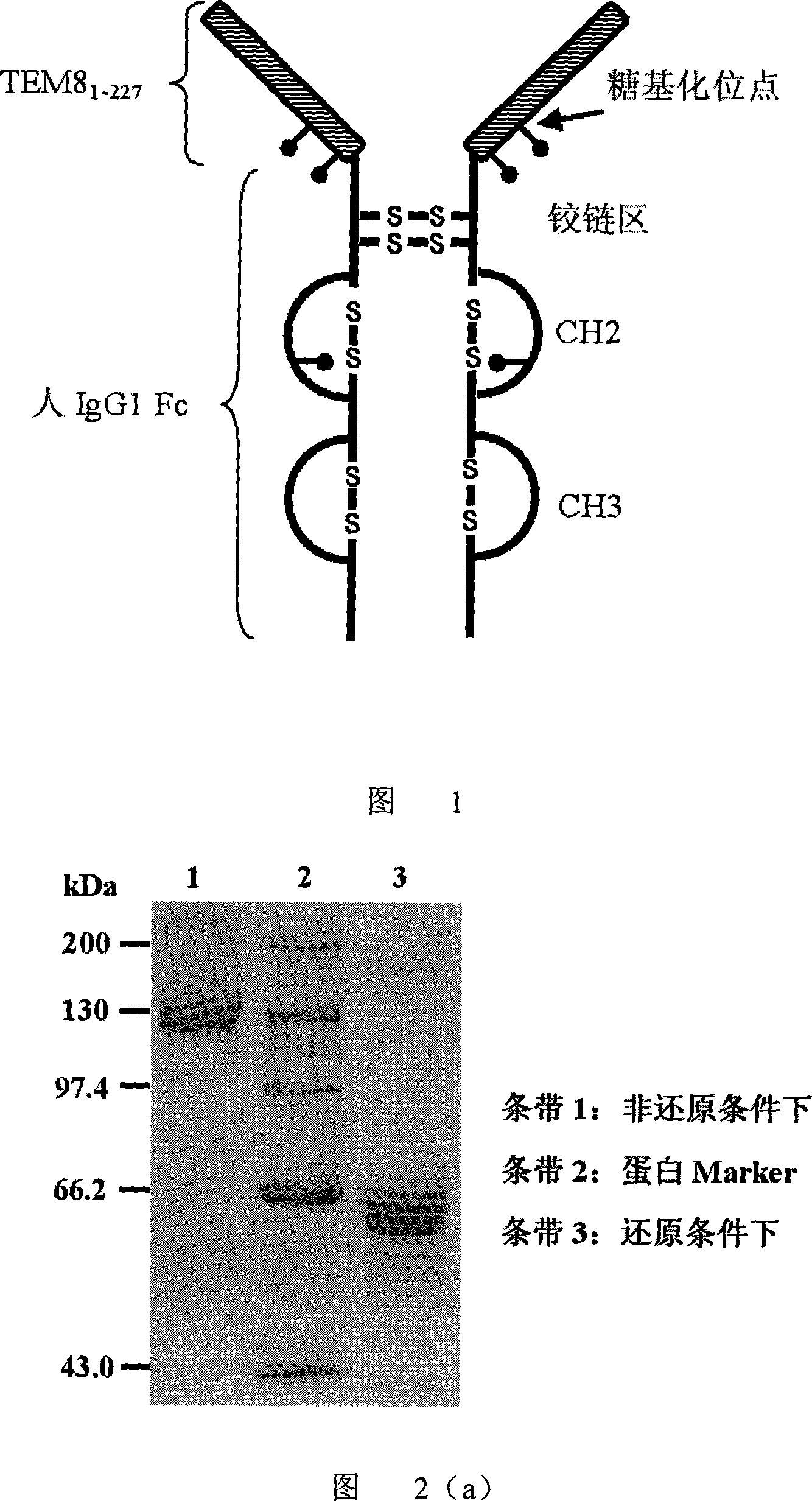
[0035] According to the method described in Chinese patent 200510084233.5, gene recombinant TEM8-Fc fusion protein was expressed in rCHO cells, and rCHO cells were cultivated with serum-free medium (Chinese patent ZL 200310124257.X), and nProtein A Sepharose 4 Fast Flow affinity column ( Amersham Biosciences) purified TEM8-Fc from the cell culture supernatant. The schematic diagram of the molecular structure of the TEM8-Fc fusion protein is shown in FIG. 1 . If TEM8-Fc can be correctly expressed in CHO cells, the expressed protein is expected to be a homodimeric antibody-like molecule, that is, the single chain of TEM8-Fc is connected into a dimer by an interchain disulfide bond at the hinge region (Figure 1 ). The single-chain TEM8-Fc molecule has a total of 459 amino acid residues, of which the first 27 amino acids are the signal peptide of TEM8, which are excised when extracellularly secreted into the ce...
Embodiment 2
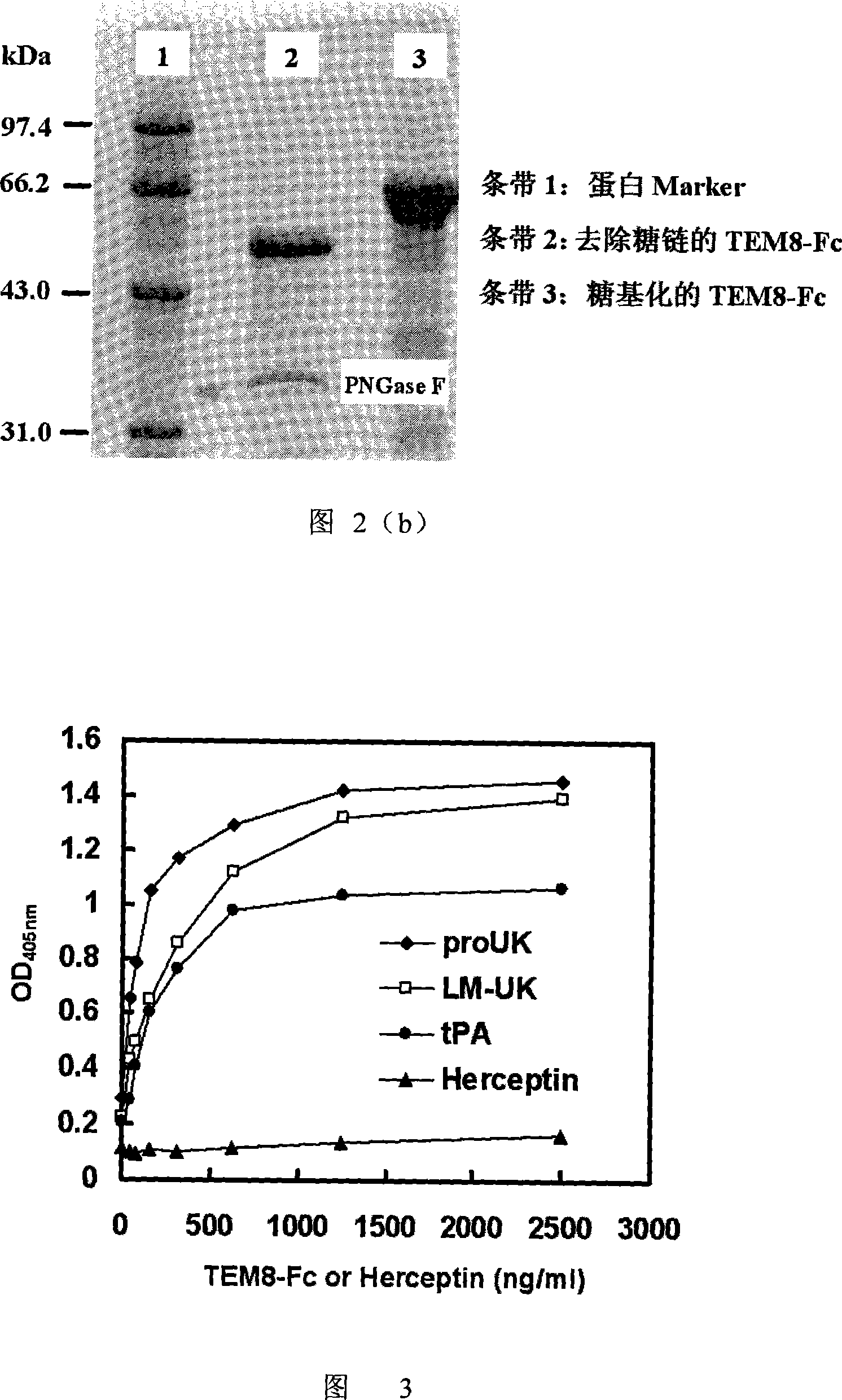
[0037] Example 2. ELISA confirmed the specific binding of TEM8 to uPA or tPA
[0038] Gene recombination of prourokinase (Pro-UK, source: Hu Xianwen, Xiao Chengzu, Li Zuohu. Pilotproduction of u-PA with porous microcarrier cell culture. Cytotechnology.2000.33(1~3): 13-19), low gene recombination Molecular weight urokinase (LMW-UK, Chinese patent: low molecular weight urokinase mutant and its expression vector, application number: 200610065813.4, publication number CN1880449) and gene recombinant tissue plasminogen activator (tPA, source: Roche company) Coat NUNCTM enzyme-linked plate at 0.8 μg / well, add TEM8-Fc fusion protein after blocking with 3% BSA, fully wash after incubation, then add HRP-labeled goat anti-human IgG (H+L) enzyme-labeled polyclonal antibody, add after incubation TMB reagent color development, OD value measured at 405nm. The humanized monoclonal antibody Herceptin (Roche) was used as a negative control. The results are shown in Figure 3. For the first ti...
Embodiment 3
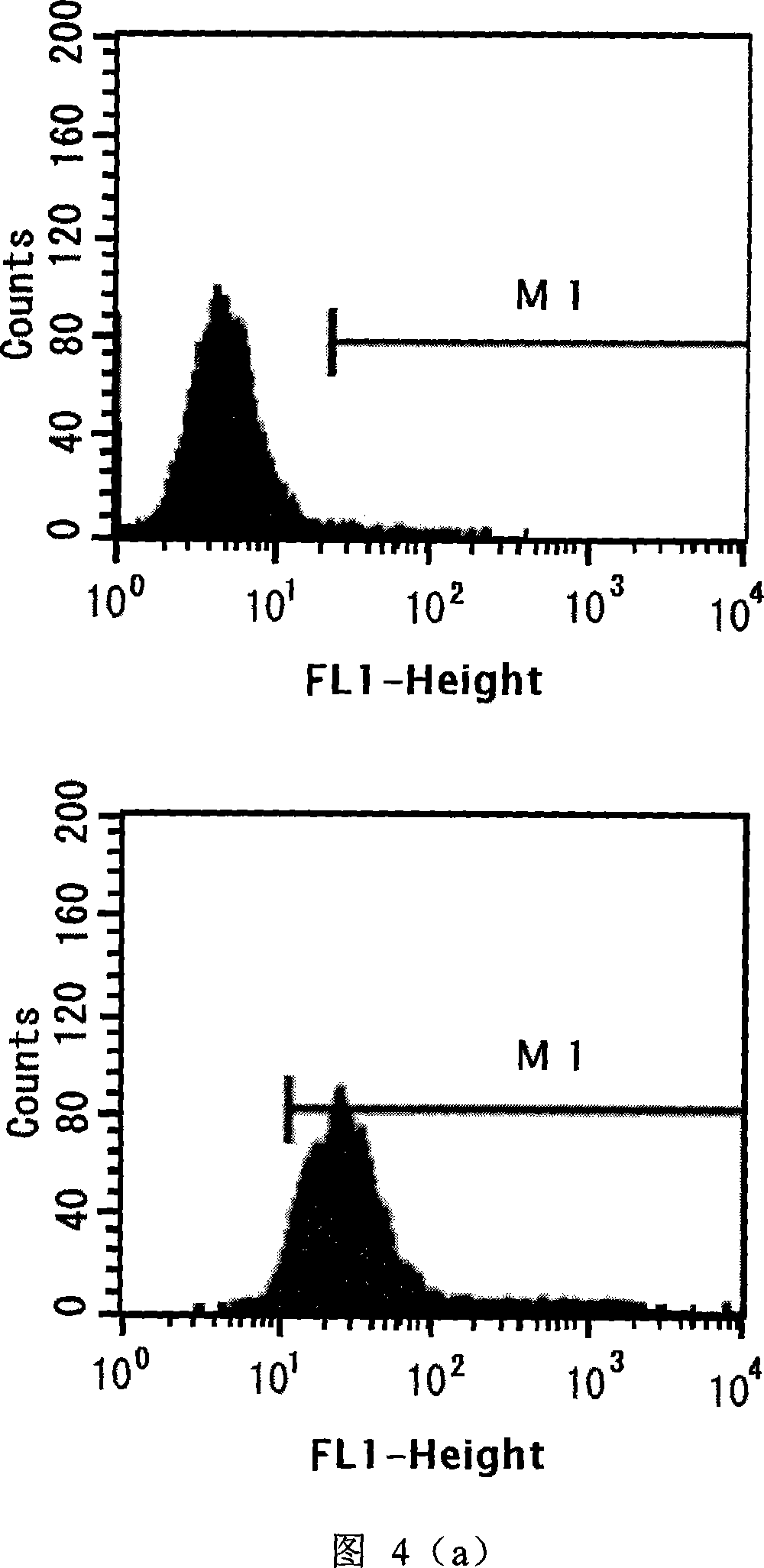
[0039] Example 3. TEM8-Fc fusion protein can inhibit the migration of tumor cells induced by uPA
[0040] Since uPA plays a pivotal role in the migration of tumor cells, blocking uPA may inhibit the migration of tumor cells. The overexpression of TEM8 on the surface of liver cancer cells HepG2 (ATCC No.HB8065) was analyzed by flow cytometry (Fig. 4(a)). The anti-TEM8 rabbit polyclonal antibody used in this experiment was purchased from Santa Cruz Company. Transwell in vitro cell migration assay (source: Corning Costar Corporation, http: / / www.tc.umn.edu / ~shimi002 / SOPtranswell.pdf) was used to verify that TEM8-Fc could block uPA to inhibit the migration of HepG2 tumor cells.
[0041] Method: HepG2 cells were divided into 1×10 per well 5 Two cells were inoculated into the upper chamber of Transwells, HepG2 cells were treated with LMW-UK (10nmol / L) for 4 hours to stimulate migration, and the blocking effect of TEM8-Fc (25g / mL) on LMW-UK was observed. After 4 hours of exposure,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com