Method for preparing recombinant virus and application of method
A recombinant virus and virus technology, applied in the field of genetic engineering, can solve the problems of complicated and tedious operation steps, difficult to control copy number, unable to insert at a fixed point, etc., and achieve the effects of simplifying screening steps, reducing the probability of causing adverse reactions, and convenient screening markers.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] 1) Construction of infectious clone p15A-H120
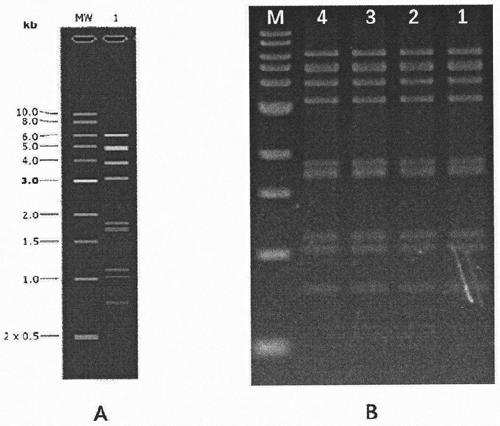
[0049] According to Genbank published three whole gene sequences (including 5'-UTR, 3'-UTR and Ploy (A)): FJ888351, FJ807652 and GU393335, the H120 whole gene sequence was divided into 4 segments and cloned into segments containing homology arms On the pBR322 vector, pBR322-H120 A~D is formed, and there are 50-70bβ overlapping regions between adjacent two segments as recombination homology arms. The first section of H120-A at the 5' end was added with a T7 promoter by PCR during the cloning process. Each fragment clone needs to be identified by enzyme digestion and sequenced to verify the correctness of the sequence, and then four fragments of DNA (H120-A~D) are obtained by enzyme digestion. Using the plasmid pUC57-HDVR-T7 ter as a template, the HDVR-T7 ter sequence containing the 3' homology arm of the genome was amplified by PCR as the fifth sequence of the H120 infectious clone. The structure of H120-A~D, HDVR-T7 ter ...
experiment example 1
[0090] Identification of rescued virus
[0091] RT-PCR detection
[0092] Take 200 μL of the rH120 F5 strain and rH120-Δ5a / H9 HA F5 virus solution to extract viral RNA according to the instructions of the Axyprep Body Fluid Virus DNA / RNA Small Extraction Kit, and dissolve the extracted RNA in 40 μL of RNase-free TE buffer. Recombinant DNase I (RNase-free) (Takara) instructions cleared DNA.
[0093] Using the above RNA as a template, primers
[0094] M-F: 5'-CCTAAGAACGGTTGGAAT-3';
[0095] M-R: 5'-TACTCTCTACACACACAC-3';
[0096] S1-F: 5'-AAGACTGAACAAAAGACCGACT-3';
[0097] S1-R: 5'-CAAAACCTGCCATAACTAACATA
[0098] -3'5ab-F:ACCACCTACACTACTTACTTG;
[0099] 5ab-R: ATTATCTGTGTGTTCCTCACAAG
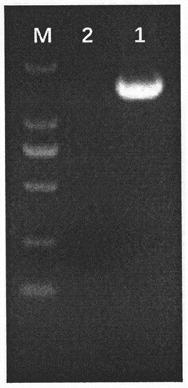
[0100] By One-Step RT-PCR Kit PrimeScript TM One Step RT-PCR Kit Ver.2 manual amplifies M, S1 and 5ab genes. The amplification results of PCR products were observed by 1% agarose gel electrophoresis, see Figure 6 , and sent to Shanghai Sangon Bioengineering Company for sequencing.
...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap