Immunologically discernible cell surface variants for use in cell therapy
A cell and surface protein technology, applied in genetically modified cells, receptors/cell surface antigens/cell surface determinants, cells modified by introducing foreign genetic material, etc., can solve the problems of on-target and off-target effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
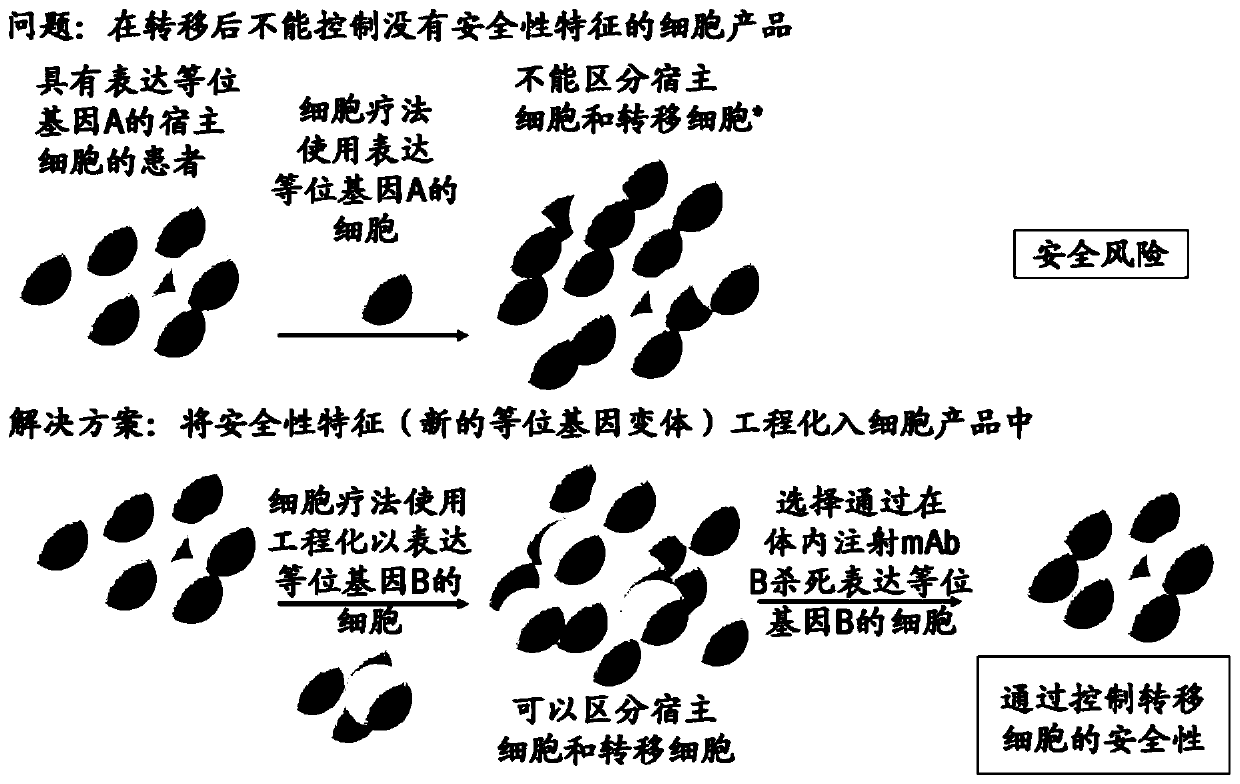
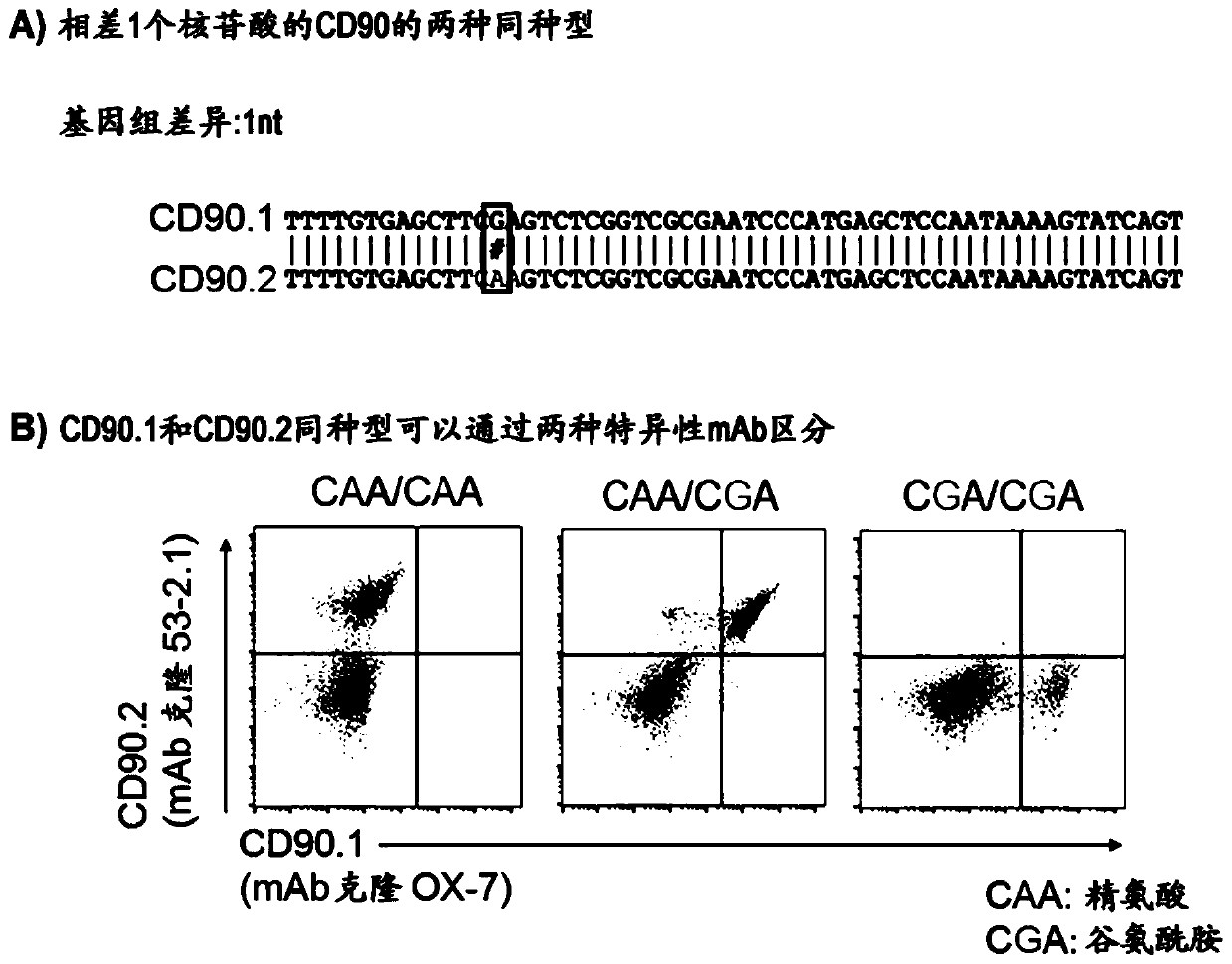
[0221] The following descriptions can be found in EP16196860.7, EP16196858.1 and PCT / EP2017 / 059799: Efficient plasmid-based gene ablation in primary T cells, targeted introduction of point mutations in primary T cells, by Enrichment of HDR-edited cells by monitoring isotype switching of alternative cell surface markers, and gene correction of mouse scurfy cells.
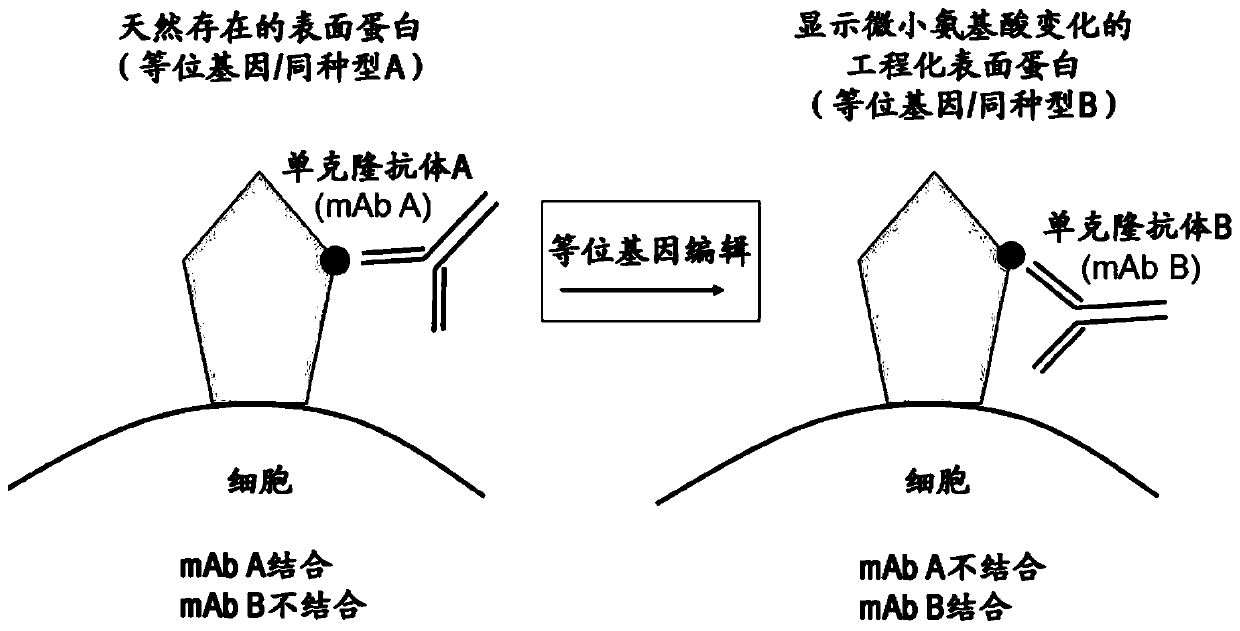
[0222] General considerations for designing engineered mutations (allele engineering):
[0223] Allelic engineering of genes by introducing site-directed small mutations (perhaps single nucleotide or single amino acid mutations) with the aim of altering the specific binding of the ligand and allowing the binding of a second specific ligand can be used as a general principle for therapeutic use. Mutations are designed in such a way that the functionality of the engineered protein is preserved as much as possible. Immunogenicity needs to be altered to enable the generation of specific binding ligands, such as monoclo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com