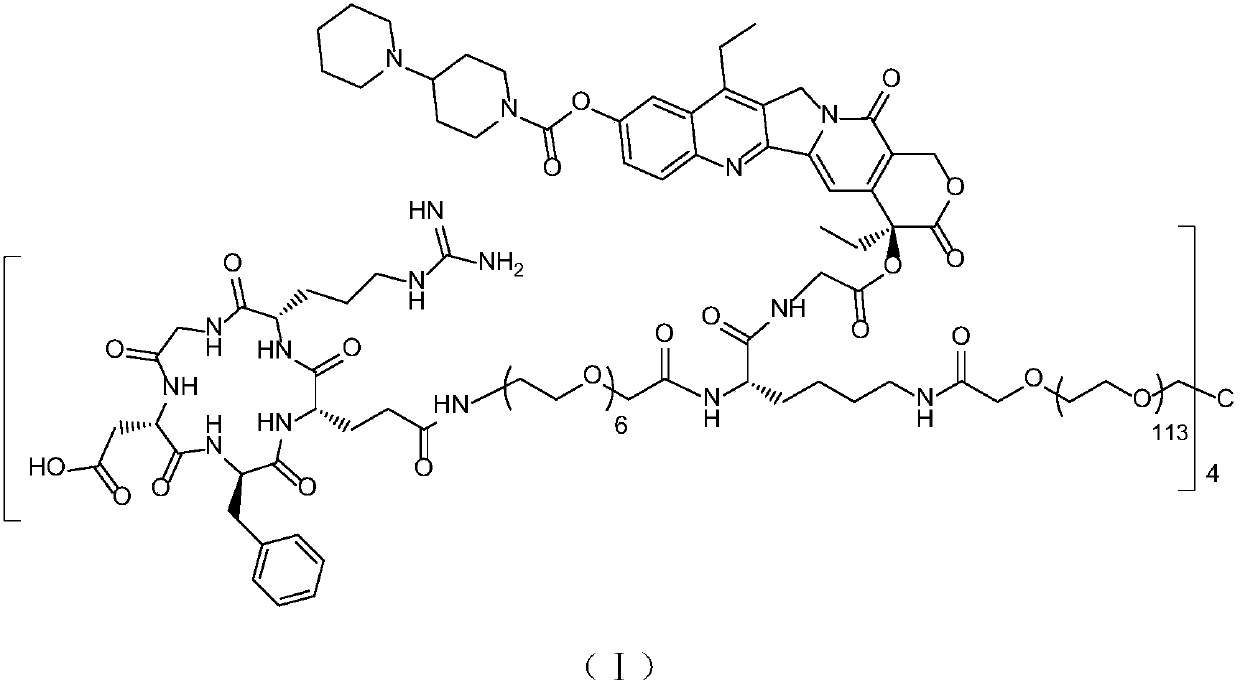
High-purity multi-arm anticancer conjugate
A high-purity, high-purity technology, applied in the field of medicine, can solve the problems of heptanesulfonic acid replacement, affecting the purity of medicines into medicinal salts, and the inability to remove heptanesulfonic acid.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The first step of purification:
[0029] Preparation column: 300DAC 300×250mm, filler: Unisil 10-300C18, mobile phase water phase: 0.02M sodium dihydrogen phosphate + 0.0075M sodium heptanesulfonate aqueous solution, mobile phase organic phase: acetonitrile, detection wavelength: 220nm;
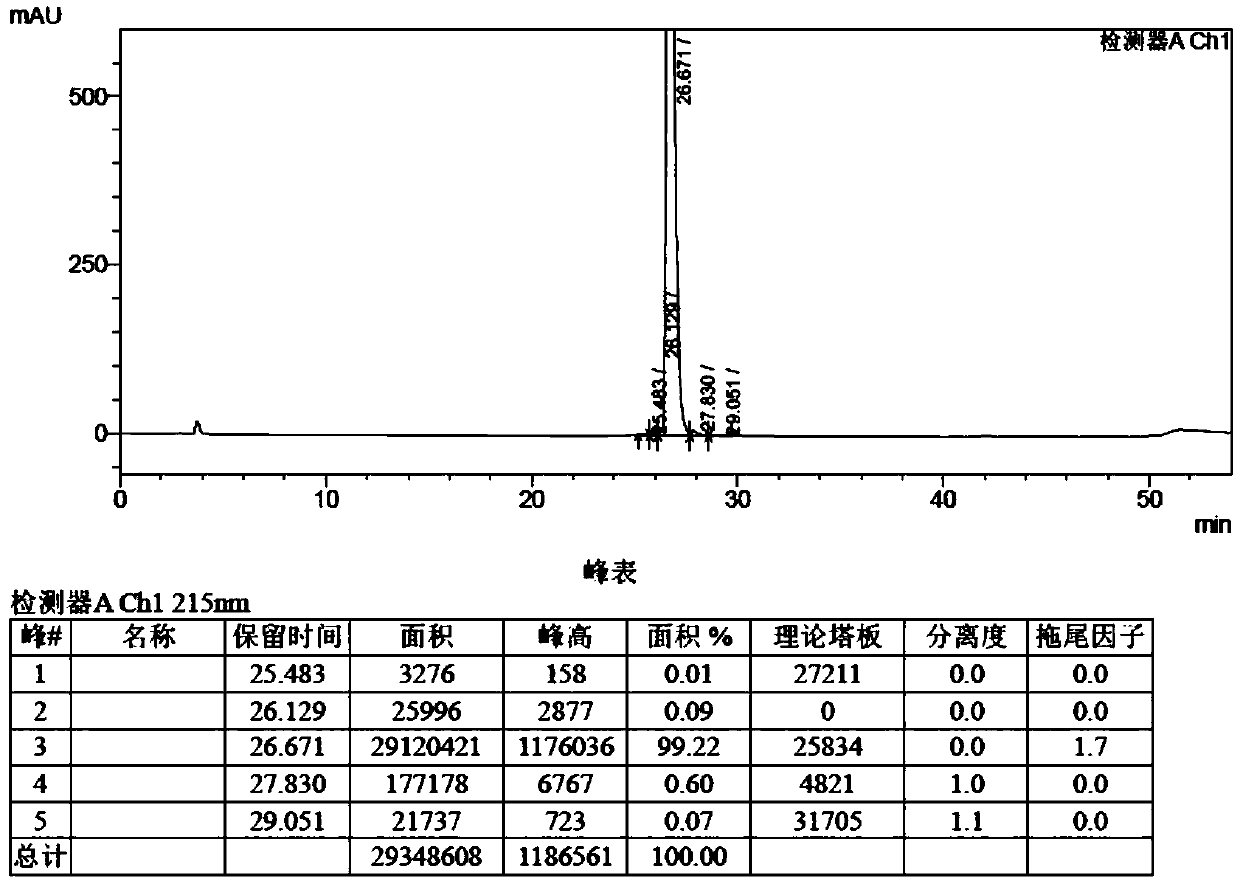
[0030] BGC0222 crude product 200g, after dissolving with 10% (V / V) acetonitrile / water, load the sample, prepare and purify by HPLC, gradient elution (time: 0~100min; mobile phase organic phase concentration: 20%~60%), the collected purity is greater than 95% % of the components as the product of the first step of purification.
Embodiment 2
[0032] Pre-desalination:
[0033] Preparation column: 300DAC, filler: Unisil 10-300C18, mobile phase aqueous phase: 5‰ acetic acid aqueous solution, mobile phase organic phase: acetonitrile, detection wavelength: 220nm;
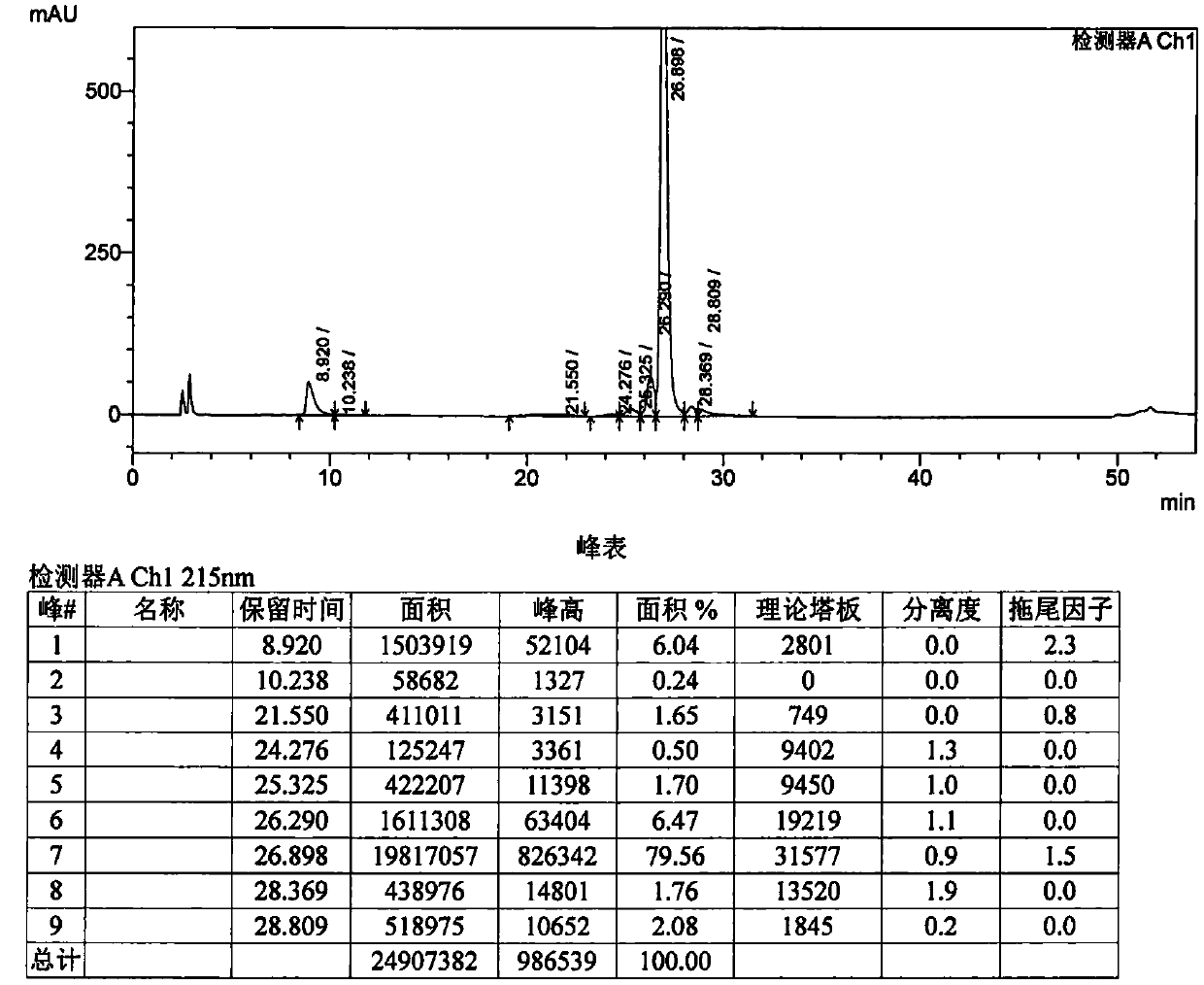
[0034] The pure products collected in the first step of purification were mixed, injected, gradient eluted (time: 0-80 min; mobile phase organic phase concentration: 5%-80%), and samples were collected.
Embodiment 3
[0036] Equilibrate and proceed to the second purification step:
[0037] Packing: anion exchange packing, model: UniGel-80Q, quantity: 2L, column: glass column (10*110cm), after the regeneration of ion exchange packing, the mobile phase washes the ion exchange column, and the mobile phase water phase is 5‰ (v / v) Acetic acid aqueous solution, mobile phase organic phase is acetonitrile, and the volume ratio of acetic acid aqueous solution and acetonitrile is 3:2, after equilibrating, load sample, use identical mobile phase ion exchange desalination, elute and collect product, obtain purity greater than 99% BGC0222.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com