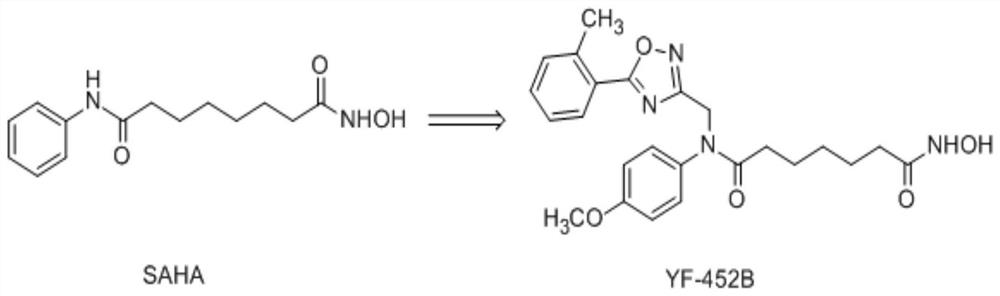
Compound, preparation method of the compound, application of the compound and products using the compound
A compound and product technology, applied in medical preparations containing active ingredients, drug combinations, organic chemistry, etc., can solve problems such as unsatisfactory inhibitory effects of histone deacetylase inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0053] The present invention also provides the preparation method of above-mentioned compound, comprises carrying out according to the reaction shown in following formula (i):
[0054]
[0055] Wherein, R1 is independently selected from one or more of the following groups: hydrogen, methyl, trifluoromethyl, fluorine, chlorine or bromine;
[0056] Preferably, after the reaction is completed, the steps of quenching, extraction, washing, drying and solvent removal are also included in sequence;
[0057] Preferably, the extracted extract is ethyl acetate, dichloromethane or ether.
[0058] Specifically, compound 2 is generated from compound 1 and bromoacetonitrile, compound 2 is reacted with acid anhydride and then esterified to generate compound 3, compound 3 is reacted with hydroxylamine hydrochloride in solvent A to obtain compound 4, and compound 4 is reacted with different substituted benzoyl chlorides Generate compound 5, compound 5 reacts with hydroxylamine hydrochlorid...
Embodiment 1
[0078] The preparation of embodiment 1 YF452B
[0079]
[0080] Compound 2 is generated from compound 1 and bromoacetonitrile, compound 2 is reacted with acid anhydride and then esterified to generate compound 3, compound 3 is reacted with hydroxylamine hydrochloride in solvent A to obtain compound 4, compound 4 is reacted with different substituted benzoyl chlorides to generate compound 5 , compound 5 reacts with hydroxylamine hydrochloride in solvent B to generate compound 6, and compound 6 reacts with methoxy to generate the target compound YF452B. After the reaction is completed, it is generally quenched with ice water and extracted with ethyl acetate, dichloromethane, ether, etc. Washing with water and saturated saline successively, drying, removing the solvent under reduced pressure at low temperature, and obtaining the final product through column chromatography, the yield varies from 30% to 70%.
[0081] The solvent A is dimethylformamide, dioxane, toluene, benzene ...
Embodiment 2
[0084] Example 2 Histone Deacetylase Inhibitor Enzyme Activity Experiment
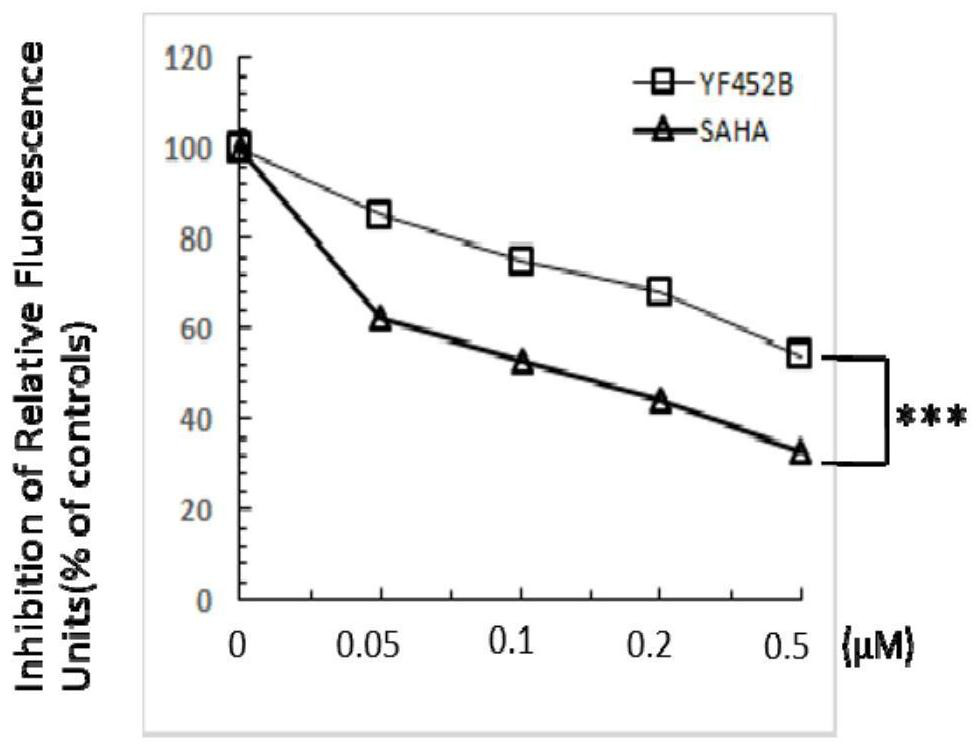
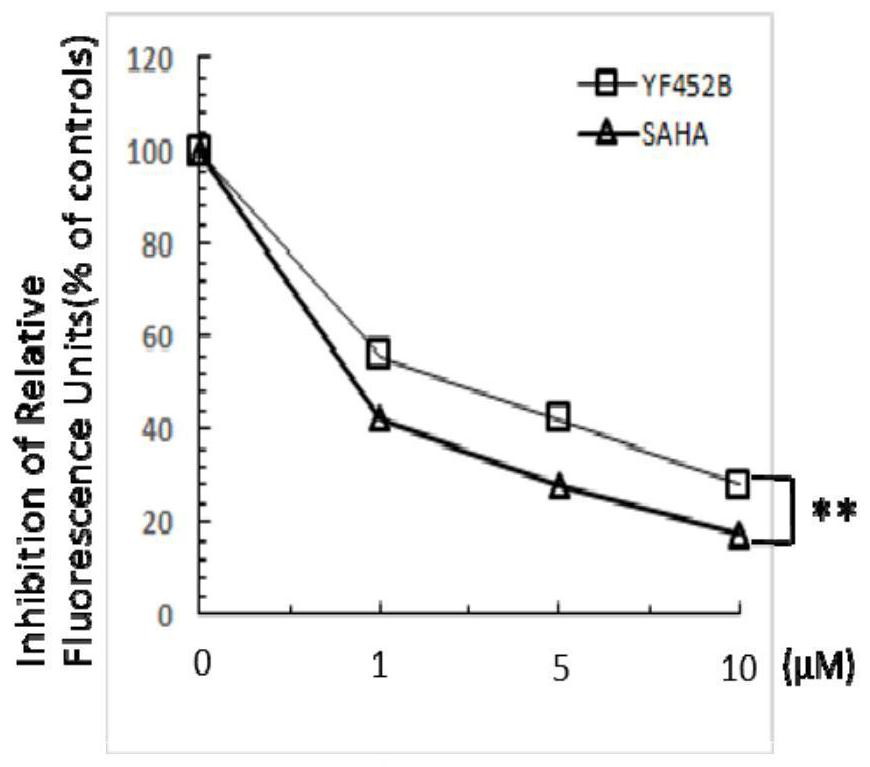
[0085]In this example, the histone deacetylase enzyme activity screening kit is used. In the experiment, the compound to be identified was mixed with histone deacetylase activity (HeLa cell or MDA-MB231 cell lysate) and histone deacetylase colorimetric substrate (containing an acetylated lysine side chain ) were incubated together. If the substrate is deacetylated, it will be activated, and then a luminescent group will be generated under the action of lysine chromogen. The final luminescent groups were read and analyzed with a microplate reader.
[0086] The result is as Figure 2A with Figure 2B As shown, the experimental results show that YF452B, similar to SAHA, can inhibit the activity of histone deacetylase in a concentration gradient-dependent manner, and at the same concentration, the inhibitory effect of YF452B on the activity of histone deacetylase is significantly better than that of S...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com