Combinations of a 4-pyrimidinesulfamide derivative with active ingredients for the treatment of endothelin related diseases
A technology of active ingredients and compositions, applied in the field of combination of 4-pyrimidinesulfonamide derivatives and active ingredients for the treatment of endothelin-related diseases, capable of solving problems such as increased side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0085] The preparation of pharmaceutical compositions can be carried out in a manner familiar to the person skilled in the art (see for example Remington, The Science and Practice of Pharmacy, 21st Edition (2005), Part 5, "Pharmaceutical Manufacturing" [published by Lippincott Williams & Wilkins]): by The crystalline forms of the present invention, optionally in combination with other therapeutically useful substances, are formulated together with suitable, nontoxic, inert, pharmaceutically acceptable solid or liquid carrier materials and (if desired) customary pharmaceutical adjuvants. In galenical form.
[0086] 23) Another embodiment relates to any one of embodiments 1) to 22), in particular to a solid medicament according to any one of embodiments 8) to 14), or according to any one of embodiments 15) to 21) Composition, especially in the form of a tablet, comprising inert microcrystalline cellulose, lactose, hydroxypropyl cellulose, croscarmellose sodium and stearic acid a...
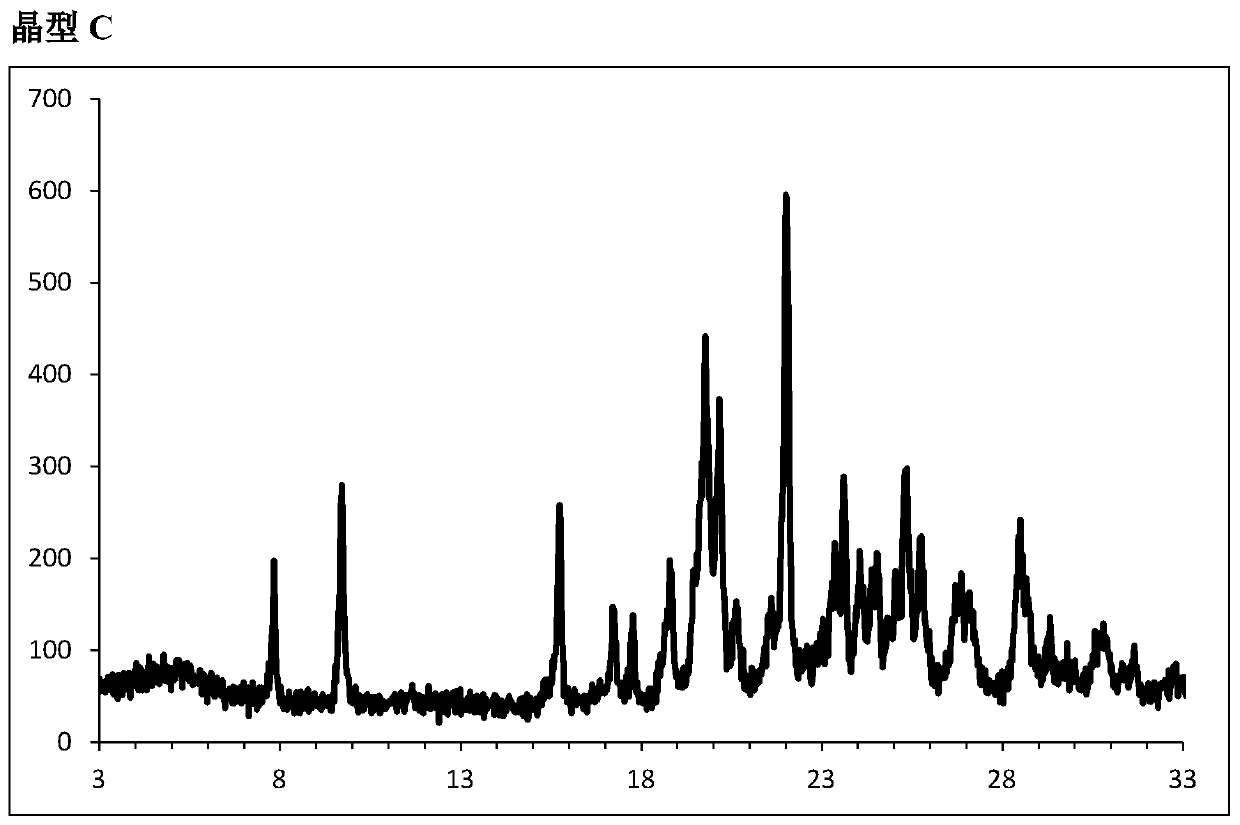
Embodiment 1
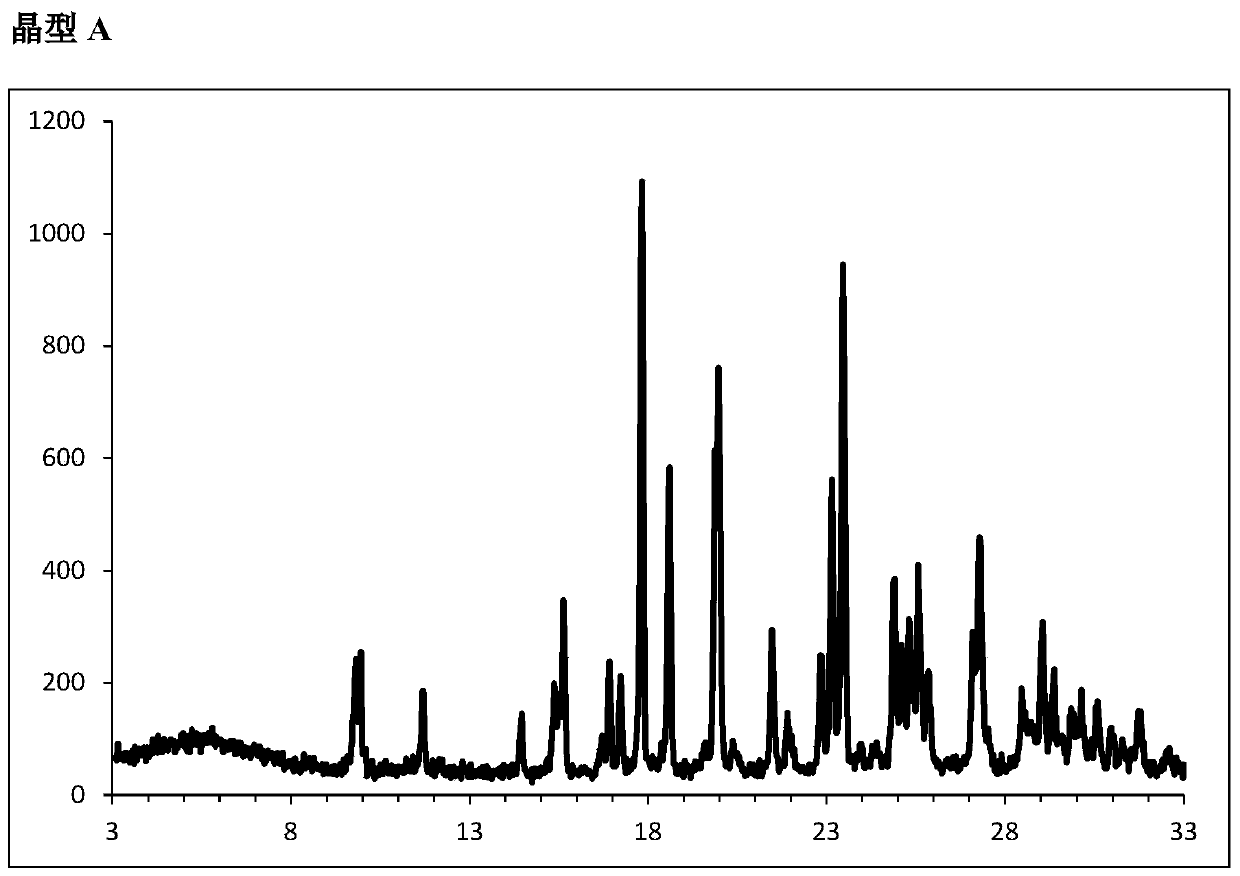
[0172] Example 1: Form A:
[0173] 1.1. Add 5-(4-bromophenyl)-4-(2-((5-bromopyrimidin-2-yl)oxy)ethoxy)-6-fluoropyrimidine ( 100g, 0.213mol, 1 equivalent), sulfonamide (40.9g, 0.425mol, 2.0 equivalent), K 2 CO 3 (147 g, 1.06 mol, 5 eq) and DMSO (500 mL, 5 vol) spiked with water (2 mL, 0.111 mol, 0.5 eq). The heterogeneous mixture was heated to about 70° C. in about 3 hours, after which time complete conversion was observed. After cooling to 20°C, most of the inorganic salt material was removed by filtration. The filter cake was washed with EtOAc / iPrOAc 1:1 (300 mL, 3 vol). Celite (100 g, 1 wt.) topped with a layer of charcoal (20 g, 0.2 wt.) was pretreated with EtOAc / iPrOAc 1:1 (500 mL, 5 vol.) (filtrate discarded). The reaction mixture was filtered through the filter cake and washed with EtOAc / iPrOAc 1:1 (300 mL, 3 vol). Then 1 M aqueous NaOAc (500 mL, 0.5 mol, 2.3 eq, 5 vol) was added while maintaining the temperature at 25-35 °C. The aqueous phase was washed a second ...
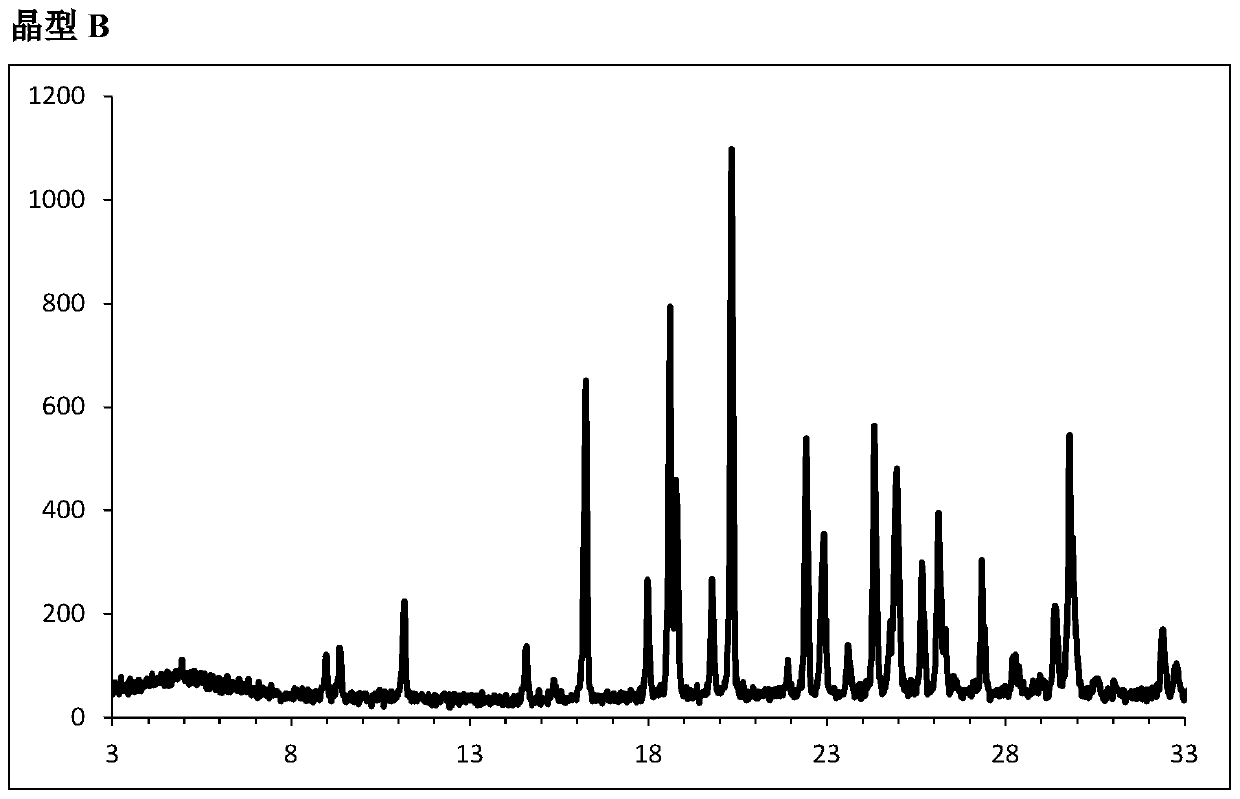
Embodiment 2
[0175] Example 2: Form B (the DCM solvate of the COMPOUND):
[0176] 5-(4-Bromophenyl)-4-(2-((5-bromopyrimidin-2-yl)oxy)ethoxy)-6-fluoropyrimidine (10.0g, 21.3mmol, 1.00eq), sulfo Amide (4.1 g, 42.5 mmol, 2.0 equiv) and K 2 CO 3 (14.7 g, 106 mmol, 5.0 equiv) was suspended in DMSO (50 mL, 5 vol) and heated to 70 °C for 5 hours. The mixture was cooled to room temperature and EtOAc (40 mL, 4 vols) was added followed by water (100 mL, 10 vols). After separation of the layers (organic phase discarded), the aqueous phase was extracted with DCM (100 mL, 10 vol). The DCM layer was acidified from pH 11.5 to pH 7.0 with concentrated AcOH (3 mL, 52 mmol, 2.5 equiv), resulting in crystallization of the product. The suspension was cooled to 0°C for 1 hour, then to -5°C for 15 minutes. The solid was filtered, washed with cold DCM (10 mL, 1 vol) and dried to give {5-(4-bromo-phenyl)-6-[2-(5-bromo-pyrimidin-2-yl-oxy)-ethyl Oxy]-pyrimidin-4-yl}-sulfonamide in DCM solvate as a white solid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com