Amino-acid biphenyl compound and medicine usage thereof
A technology of compounds and amino acids, applied in the field of medicinal chemistry, can solve the problems of low oral bioavailability, toxicity, and easy explosion.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
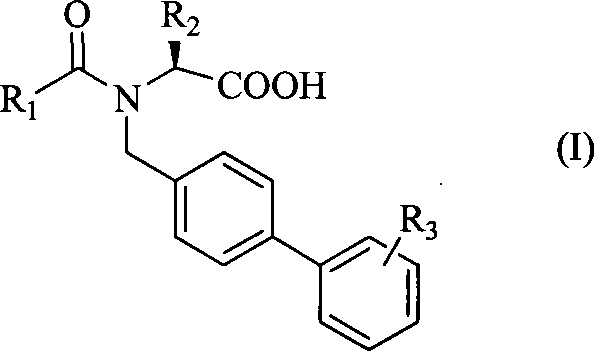
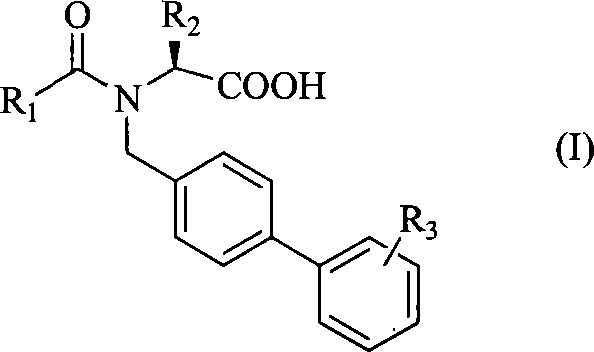
[0033] [Example 1] N-[4-(2-(5-oxo-1,2,4-oxadiazole)phenyl)benzyl]-N-butyryl-L-valine
[0034] Step 1: (L)-Valine Methyl Ester Hydrochloride
[0035]Add 2 mL of anhydrous methanol to a 50 mL three-necked flask, stir, add 0.615 mL of thionyl chloride (8.55 mmol) and 1 g (L)-valine (8.55 mmol), stir for 2 h, and evaporate the solvent. Recrystallized from methanol-ether (1:15) to obtain 1.35 g of a colorless needle-like solid, yield 94.8%, mp: 128-132°C.
[0036] Step 2: N-[4-(2-cyanophenyl)benzyl]-L-valine methyl ester
[0037] Dissolve 2.0g (L)-valine methyl ester hydrochloride (12.0mmol) in 30mL DMF, stir, add 1.53mL triethylamine (12.0mmol), then add 3.0g 2'-cyano-4- Bromomethylbiphenyl (11 mmol). React at 50°C for 1 h, add 15 mL of distilled water, and extract with ethyl acetate (20 mL×2). Combine the organic phases, saturate with KHCO 3 The solution was washed with 30 mL×2, dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure to obt...
Embodiment 2
[0048] [Example 2] N-[4-(2-(5-oxo-1,2,4-oxadiazole)phenyl)benzyl]-N-pentanoyl-L-valine
[0049] The experimental procedure is as described in Example 1, and the yield is 82.4%.1 H-NMR (CDCl 3 , 500.12MHz, doubling due to amide rotamers) δ: 7.64-7.22 (m, 8H, Ph-H), 4.70 (d, J=16.0Hz), 4.22-4.10 (m, 2H, -N-CH 2 -), 4.02-3.72(m, 1H, -CHN-), 2.75-2.38(m, 3H, -C H CH 3 ,-CO-CH 2 -), 1.78-1.68 (m, 2H, -CH 2 -C H 2 -CH 2 ), 1.49-1.25 (m, 2H, -CH 2 -C H 2 -CH 3 ), 1.01-0.87(m, 9H); MS(m / z): 452.3[M+1] + , 474.3[M+Na] + .
Embodiment 3
[0050] [Example 3] N-[4-(2-(5-oxo-1,2,4-oxadiazole)phenyl)benzyl]-N-butyryl-L-phenylalanine
[0051] The experimental procedure is as described in Example 1, and the yield is 89.4%. 1 H-NMR (CDCl 3 , 500.12MHz, doubling due to amide rotamers) δ: 7.61-7.41 (13H, m, Ph-H), 4.43 (d, 1H, J=14.2Hz, -N-CH-), 4.30-4.27 (m), 3.63(d, J=16.3Hz, -CH 2 -N-), 3.27(d, 2H, J=7.8Hz, -CH-C H 2 -Ph), 2.31-2.27(m, 2H, -CO-CH 2 -), 1.31-1.28 (m, 2H, -CH 2 -C H 2 -CH 3 ), 0.91-0.85 (m, 3H, -CH 3 ); MS(m / z): 486.1[M+1] + , 508.2[M+Na] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com