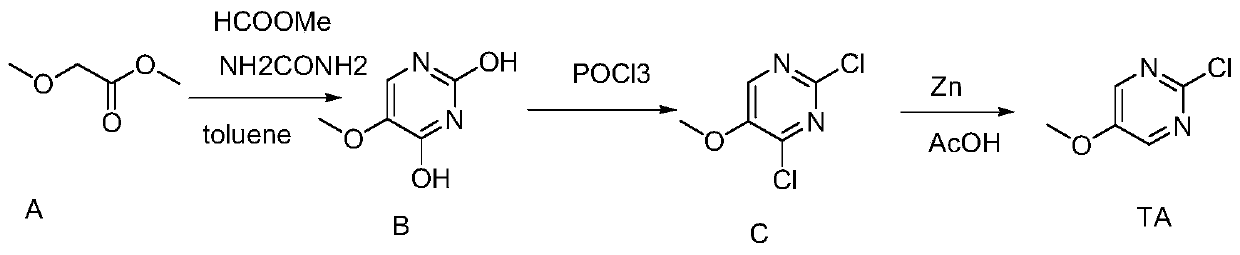
Preparation method of 2-chloro-5-methoxypyrimidine
A technology of methoxypyrimidine and toluene, which is applied in the field of compound preparation, can solve the problems of low product yield, high cost, complicated method of 2-chloro-5-methoxypyrimidine, etc., and achieve high product purity and mild reaction , easy-to-control effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0012] In order to enable those skilled in the art to better understand the technical solutions of the present invention, the present invention will be further described in detail below in conjunction with the accompanying drawings and preferred embodiments.
[0013] As shown in the figure, the present invention comprises the following steps:
[0014] a. the compound shown in preparation formula B:
[0015] Put 3.5L of toluene and 850g of sodium methoxide into a 10L reaction flask, cool down to 10°C with ice water, and add 606g of methyl formate dropwise. Since the boiling point of methyl formate is low, keep the temperature of the reaction solution lower than 20°C, after the dropwise addition, stir for 30 minutes;
[0016] Then add 750g of methyl methoxyacetate dropwise. During the dropwise addition, the temperature of the reaction solution is lower than 30°C. After the dropwise addition is completed, stir for 5-6 hours. After the stirring is completed, let stand overnight; ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com