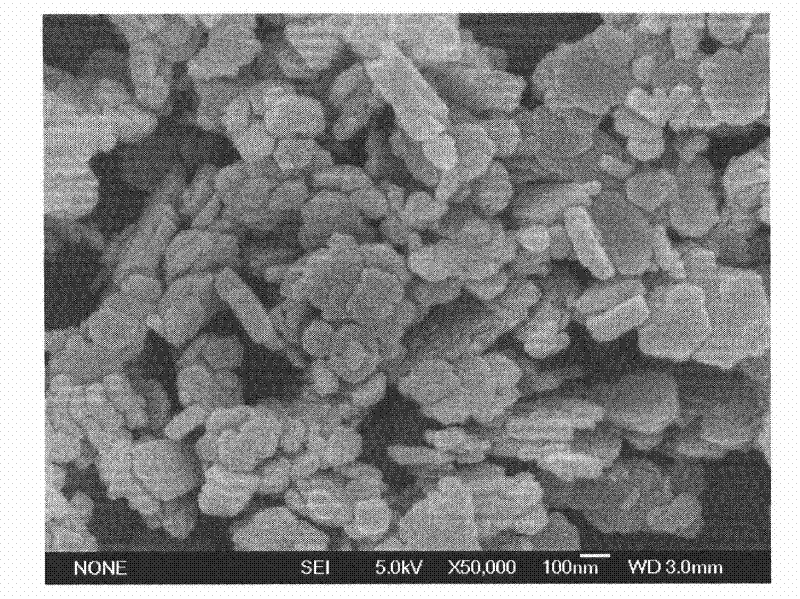
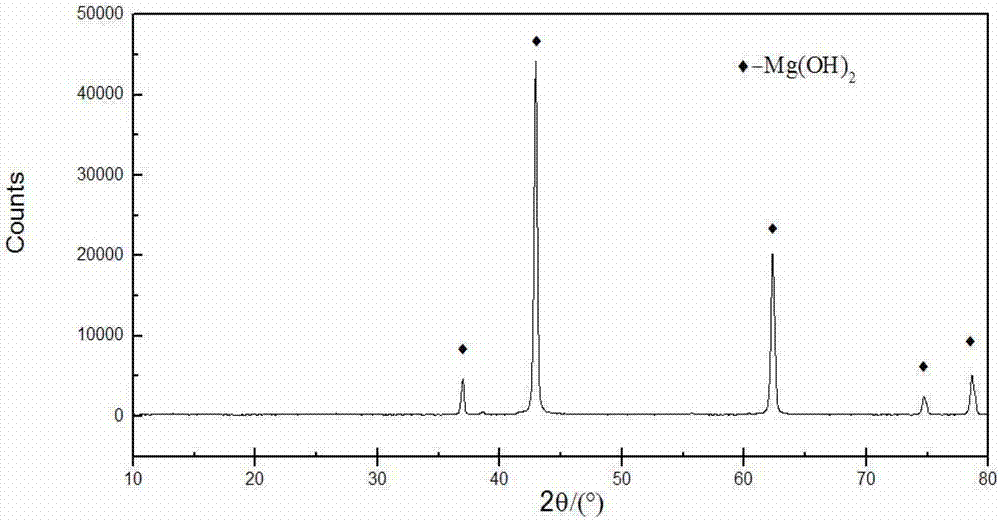
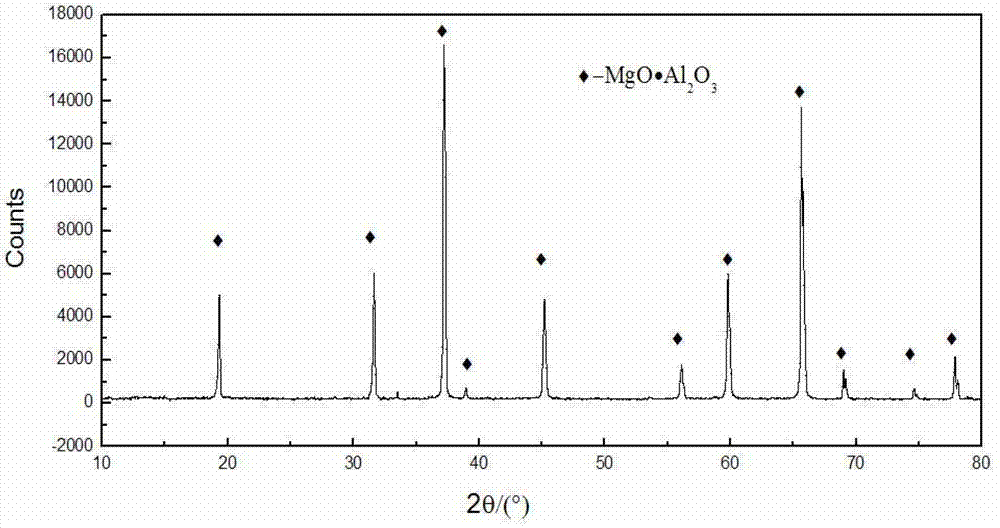
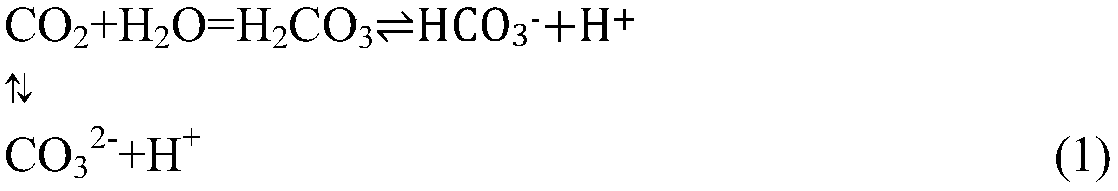Patents
Literature
256 results about "Magnesium carbonate hydroxide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Calcium carbonate and magnesium hydroxide is a combination antacid used to treat indigestion, upset stomach, and heartburn. Calcium carbonate and magnesium hydroxide may also be used for purposes not listed in this medication guide.
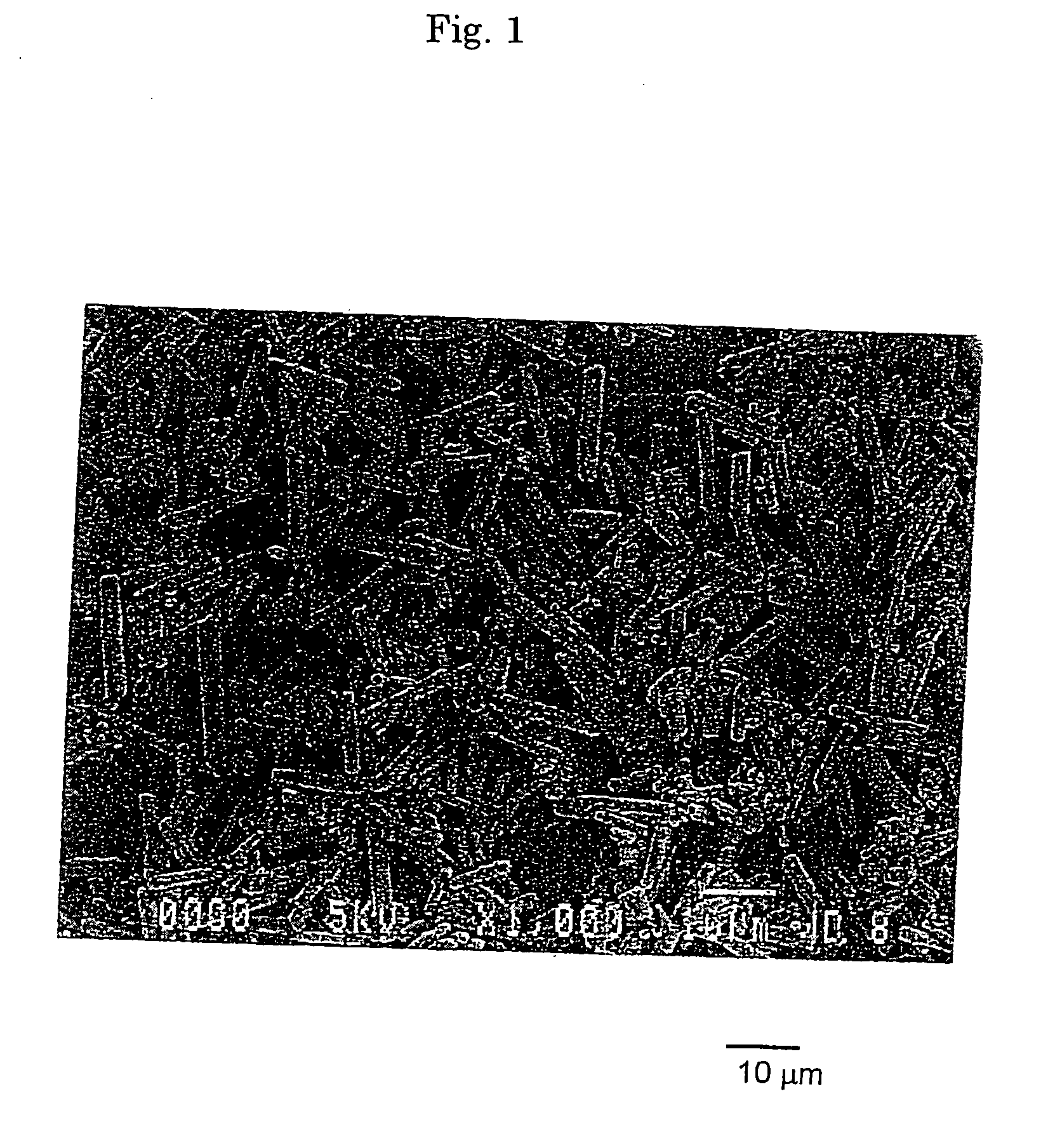
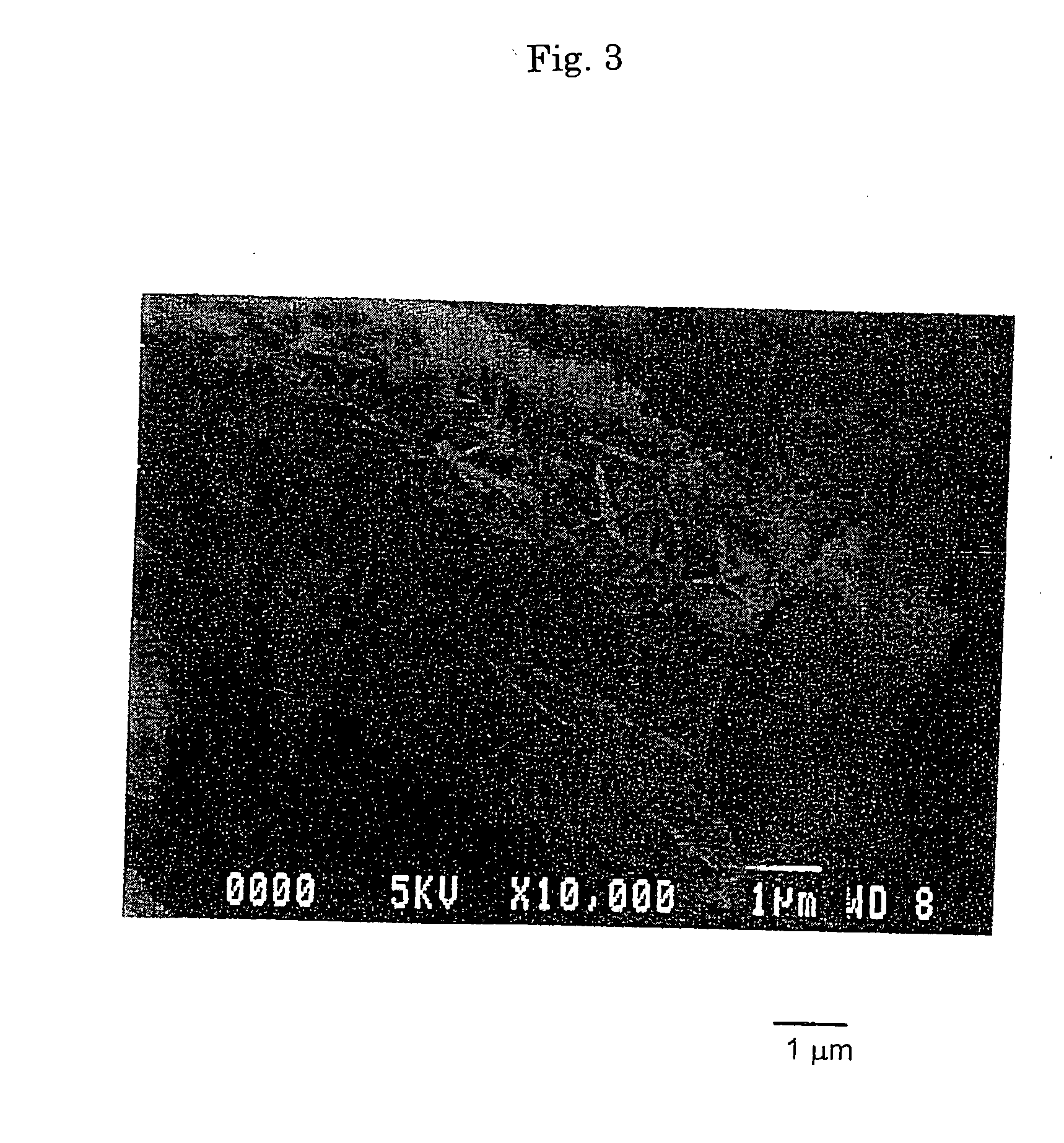
Basic magnesium carbonate, production method and use of the same
ActiveUS20050129606A1Large specific surface areaImprove featuresCalcium/strontium/barium carbonatesBiocideCarbonateMagnesium carbonate hydroxide
Owner:NITTETABU MINING CORP
Method for preparing nitrogen doping hollow carbon nanocages
InactiveCN102530922AHigh purityHigh mesoporosityMaterial nanotechnologyNano-carbonPtru catalystCarbonate
The invention relates to a method for preparing nitrogen doping hollow carbon nanocages, which comprises the following steps that: (1) basic magnesium carbonate or magnesium carbonate is taken to be added into a reaction tube and is uniformly dispersed, then the reaction tube is placed in a tube furnace, air in the tube furnace is extracted, inert gases are filled into the tube furnace, under the condition of 10-500sccm of inert gases, the temperature of the tube furnace is increased to 650-1,100 DEG C, then the inert gas flow is guided into steam which contains carbon (C) and nitrogen (N), and after reacting for 5-240min, the temperature of the tube furnace is reduced to a room temperature; and (2) powder in the reaction tube is collected, put into hydrochloric acid or sulfuric acid solution to be soaked for 5-720min, filtered, cleaned by deionized water and dried, so a nitrogen doping hollow carbon nanocage is obtained. The nitrogen doping hollow carbon nanocage which is produced by the method has the advantages of high specific surface area, large pore volume, high mesoporous ratio, good graphitization degree and the like, and is a metal-free oxygen reduction reaction catalyst with excellent performance.
Owner:NANJING UNIV
Fungicidal and parasiticidal fire-retardant powder
InactiveUS20150368560A1Reduce weightReduce the amount requiredFireproof paintsBiocidePhosphateFire retardant
Fire-retardant powder comprising at least 30% by weight of mono ammonium dihydrogen phosphate and / or di-ammonium monohydrogen phosphate, at least 5% by weight of alkaline bicarbonate, at least 3% by weight of silica, and at least 5% by weight of a compound selected from the group consisting of: sodium chloride, potassium chloride, potassium bromide, potassium sulfate, magnesium carbonate hydroxide pentahydrate, magnesium chloride hexahydrate, iron(II) sulfate heptahydrate, zinc (II) chloride, and combinations thereof. The invention also relates to building materials preferably comprising natural fibers and comprising at least 5% by weight, and at most 30% of a powder according to the invention.
Owner:SOLVAY SA
Process for producing magnesium hydroxide and calcium carbonate by dolomite conversion method
InactiveCN102225775AReduce consumptionGood environmental benefitsCalcium/strontium/barium carbonatesMagnesium hydroxideEvaporationCarbonization
The invention provides a process for producing magnesium hydroxide and calcium carbonate by the dolomite conversion method. The process comprises the following steps: calcining dolomite, carrying out a digestion reaction on dolomite and water and refining so as to obtain a refined slurry of calcium hydrate and magnesium hydroxide; reacting the slurry with an ammonium chloride solution to enable calcium hydrate to react with ammonium chloride; carrying out ammonia evaporation so as to obtain a fluid suspension of a calcium chloride solution, ammonia gas and magnesium hydroxide; carrying out filtering and washing so as to obtain a magnesium hydroxide product; carrying out a carbonization reaction on the calcium chloride aqueous solution which has absorbed ammonia gas with kiln gas generated by the calcination of dolomite so as to obtain calcium carbonate; carrying out filtering and washing so as to obtain a calcium carbonate product. In the invention, washing water is used for the digestion of dolomite ashes so as to totally separate magnesium from calcium in dolomite and produce magnesium hydroxide and calcium carbonate products, thereby fully utilizing dolomite. The process provided in the invention enables cyclic utilization of the intermediate product ammonium chloride and no discharge of the three wastes, being in accordance with the developmental requirements for green chemicals in modern society.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
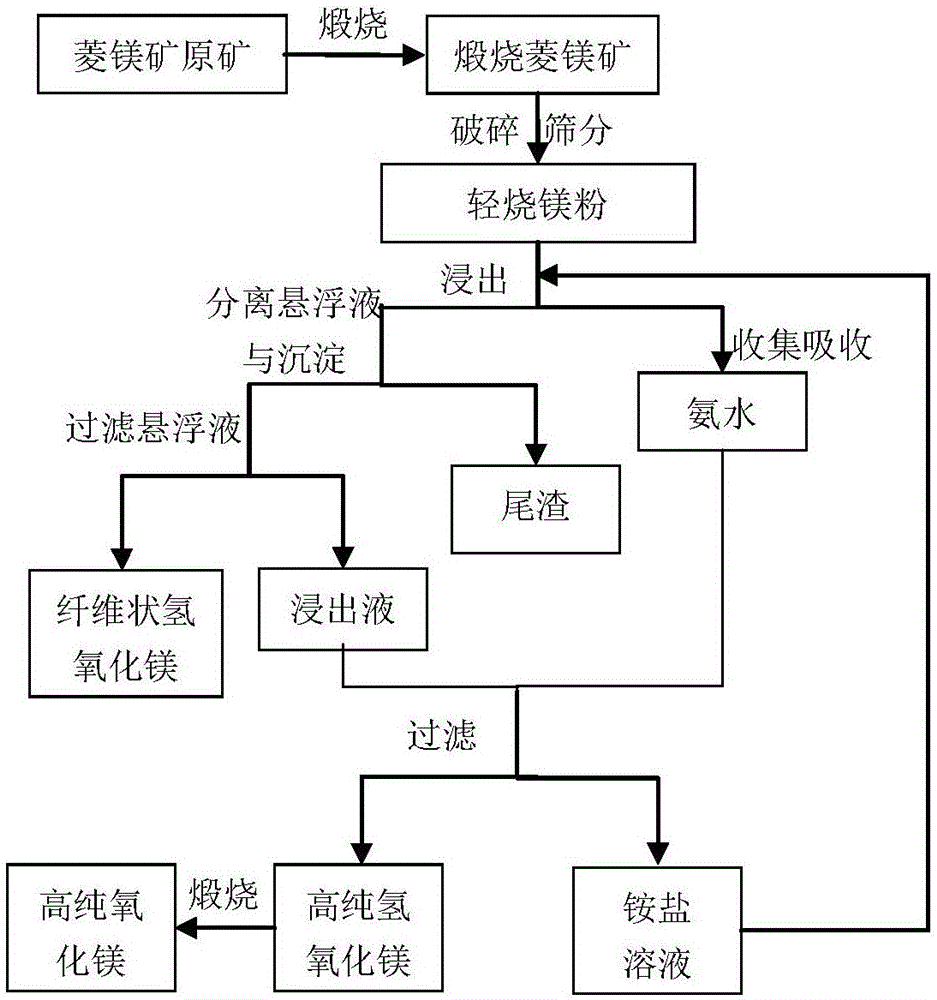
Method of producing high-purity magnesium hydroxide and magnesium oxide by low-grade magnesite
InactiveCN103011630AReduce pollutionTake advantage ofMagnesium hydroxideLime productionStrong acidsMagnesite
The invention relates to the field of mineral resource processing technology, in particular to a method of producing high-purity magnesium hydroxide and magnesium oxide by low-grade magnesite. The method comprises the following steps: calcining low-grade magnesite in a calcining furnace at calcining temperature to obtain light-calcining magnesite ore and carbon dioxide, recovering the carbon dioxide, and crushing the calcined light-calcining magnesite ore. By utilizing a method of realizing leaching, purification and separation coupling of the low-grade magnesite and producing high-pruity magnesium hydroxide and magnesium oxide through recycling of ammonia-ammonium slat, the advantages of preparing high-purity products by adopting the traditional bittern-ammonia process can be fully achieved, the method is of green and cycling clean production technique method, no wastes are emitted in the whole process, resources can be comprehensively utilized, the utilization rate of the magnesite resource can be improved, strong acid is not consumed in the technique process, other constitutions are fully utilized, the pollution to the environment can be reduced, and the method provides a new approach for reasonably developing and utilizing the low-grade magnesite resources.
Owner:新疆蓝天镁业股份有限公司
Method for producing magnesium hydroxide and calcium carbonate in manners of burning dolomite and dissolving into water to separate calcium and magnesium
ActiveCN103738986ASimple processLow costCalcium/strontium/barium carbonatesMagnesium hydroxideCalcium hydroxideCalcium carbonate precipitation
The invention provides a method for separating calcium and magnesium and preparing magnesium hydroxide by water dissolving and leaching of burnt dolomite. The method comprises the following operation steps: crushing the dolomite and burning into dolomite ashes; slaking the dolomite ashes by water to generate calcium hydroxide and magnesium hydroxide; filtering and separating an insoluble substance magnesium hydroxide; introducing carbon dioxide to leaching liquid to separate out calcium carbonate precipitate; separating again to obtain a calcium carbonate product, adding a purifying agent to filter residue to remove impurities such as iron, aluminum and the like, centrifuging and separating, and baking, so as to obtain the magnesium hydroxide product. The method has the characteristics of being low in cost, simple in process, and good in product quality, and a new production way is provided for preparation of magnesium hydroxide from dolomite.
Owner:贵州胜威化工新材料研究院有限公司
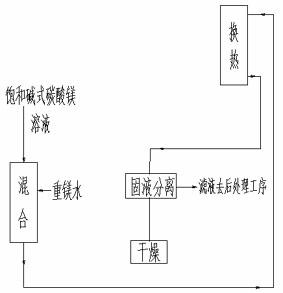
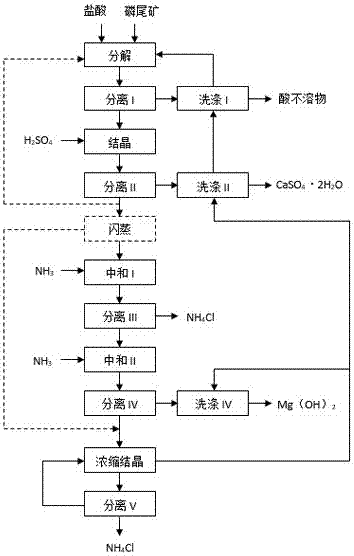
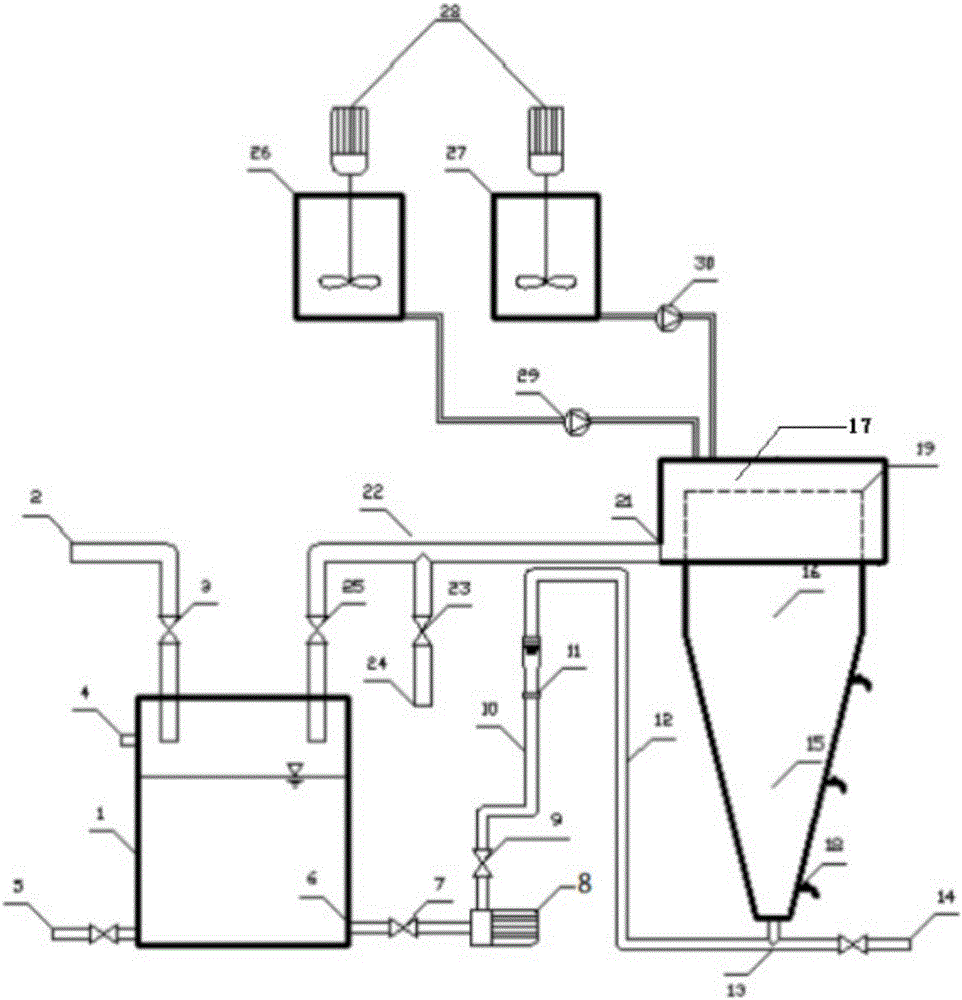
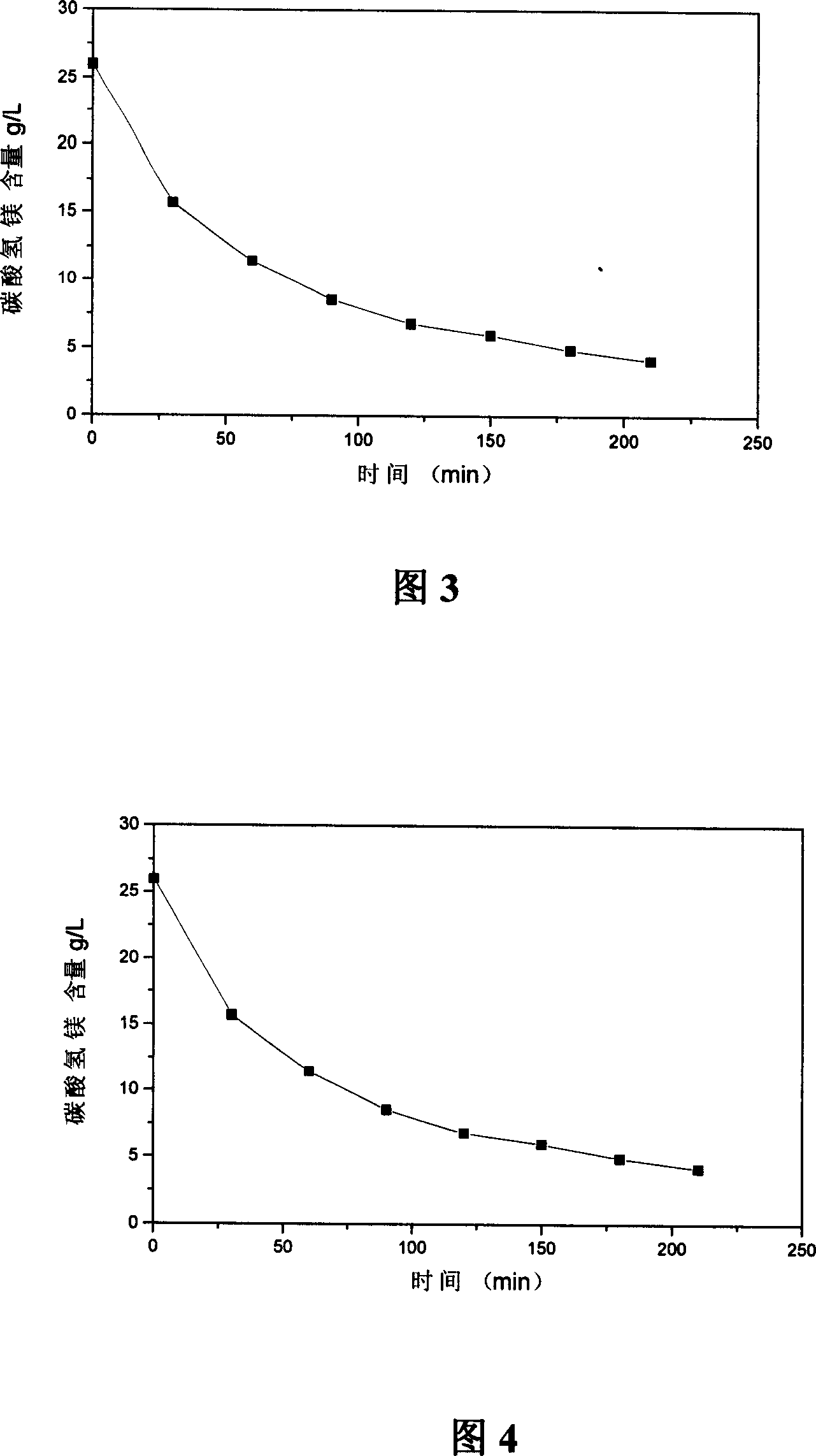
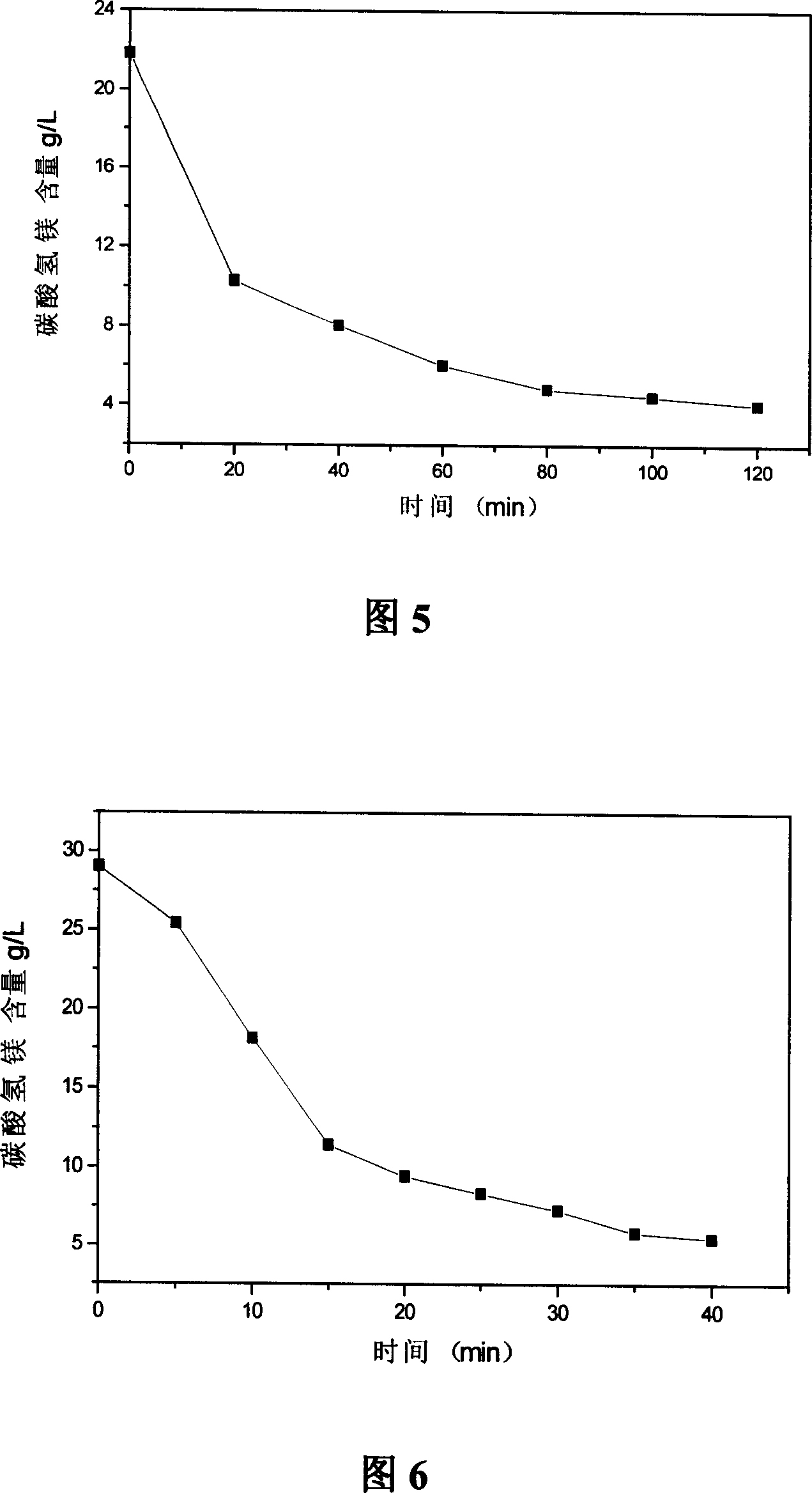
Method and device for preparing basic magnesium carbonate from heavy magnesium carbonate water by pyrolysis
ActiveCN102659147ALess investmentGuaranteed uptimeMagnesium carbonatesMagnesium bicarbonateAqueous solution
The invention relates to a method for preparing basic magnesium carbonate from heavy magnesium carbonate water by pyrolysis, which comprises the following steps: mixing a magnesium bicarbonate water solution, of which the temperature is 20-60 DEG C and the mass concentration is 3.5-100g / L, and a saturated basic magnesium carbonate or saturated basic magnesium carbonate solution containing small amount of solid crystals, of which the temperature is 40-90 DEG C, in a volume ratio of 1:(0.05-29); carrying out heat exchange in a 40-90 DEG C pyrolyzer for 10-240 minutes; carrying out solid-liquid separation on the reaction liquid to obtain solid basic magnesium carbonate crystals and a filtrate; and drying the solid basic magnesium carbonate crystals to obtain the heavy magnesium carbonate. The method provided by the invention can overcome the defects of high fuel consumption, high power consumption, high scaling tendency of the pyrolyzer or boiler, short operating cycle, incapability of long-cycle smooth operation, and the like in the existing methods.
Owner:丁丽芳 +1
Colibacillus capable of generating succinic acid by utilizing synthetic medium pure anaerobic growth and application thereof
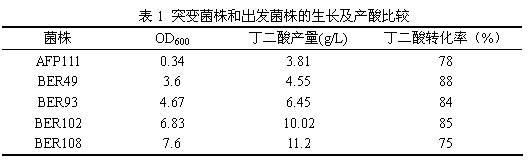
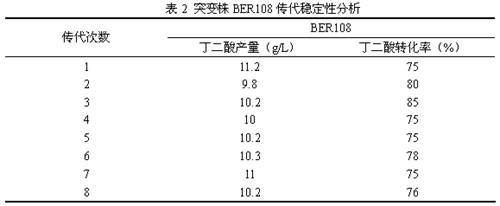
InactiveCN102643770AGreat social significanceSignificant economyBacteriaMicroorganism based processesBiotechnologyEscherichia coli
The invention relates to a colibacillus capable of generating succinic acid by utilizing synthetic medium pure anaerobic growth. The colibacillus is classified and named as EscherichiacoliBER108 and has the preservation number of CCTCC (China Center for Type Culture Collection) NO.M2012068. The invention further discloses an application of the colibacillus to fermentation production of succinic acid. According to the invention, plasma is utilized to induce colibacillus, a synthetic medium flat screen is utilized to screen out a strain capable of growing rapidly under an anaerobic condition, and under the anaerobic condition, the strain grows by utilizing an inorganic nitrogen source and glucose and accumulates succinic acid; the strain is fermented in a shake flask for 72 hours by utilizing basic magnesium carbonate as a pH regulator; the OD600 of the strain reaches 7.6, the succinic acid yield reaches 11.2g / L, the yield of the succinic acid is increased by nearly three times compared with that of an original strain; and the original strain grows slowly and is low in acid yield under the pure anaerobic synthetic medium condition, so that the mutant strain BER108 has great social significance and economic value.
Owner:NANJING UNIV OF TECH
Chewing tablet of proton pump inhibitor
InactiveCN101066279AEffective in timeImprove medication complianceOrganic active ingredientsDigestive systemSodium bicarbonateDuodenal ulcer
The present invention relates to one kind of chewing tablet of proton pump inhibitor for treating gastric ulcer, duodenal ulcer, stomal ulcer and other indications. The chewing tablet contains proton pump inhibitor in 1-5 wt%, carbonate in 35-60 wt% and hydroxide in 35-60 wt%. The proton pump inhibitor is one selected from omeprazole, S-omeprazole, pantoprazole, lansoprazole, rabeprazole, leminoprazole, tenatoprazole and their salts. The biologically acceptable buffering agent is selected from sodium bicarbonate, sodium carbonate, magnesium carbonate, calcium carbonate and their mixture, preferably sodium bicarbonate. The hydroxide is selected from magnesium hydroxide, calcium hydroxide, aluminum hydroxide, sodium hydroxide and their mixture, preferably magnesium hydroxide.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
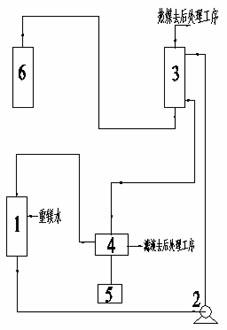
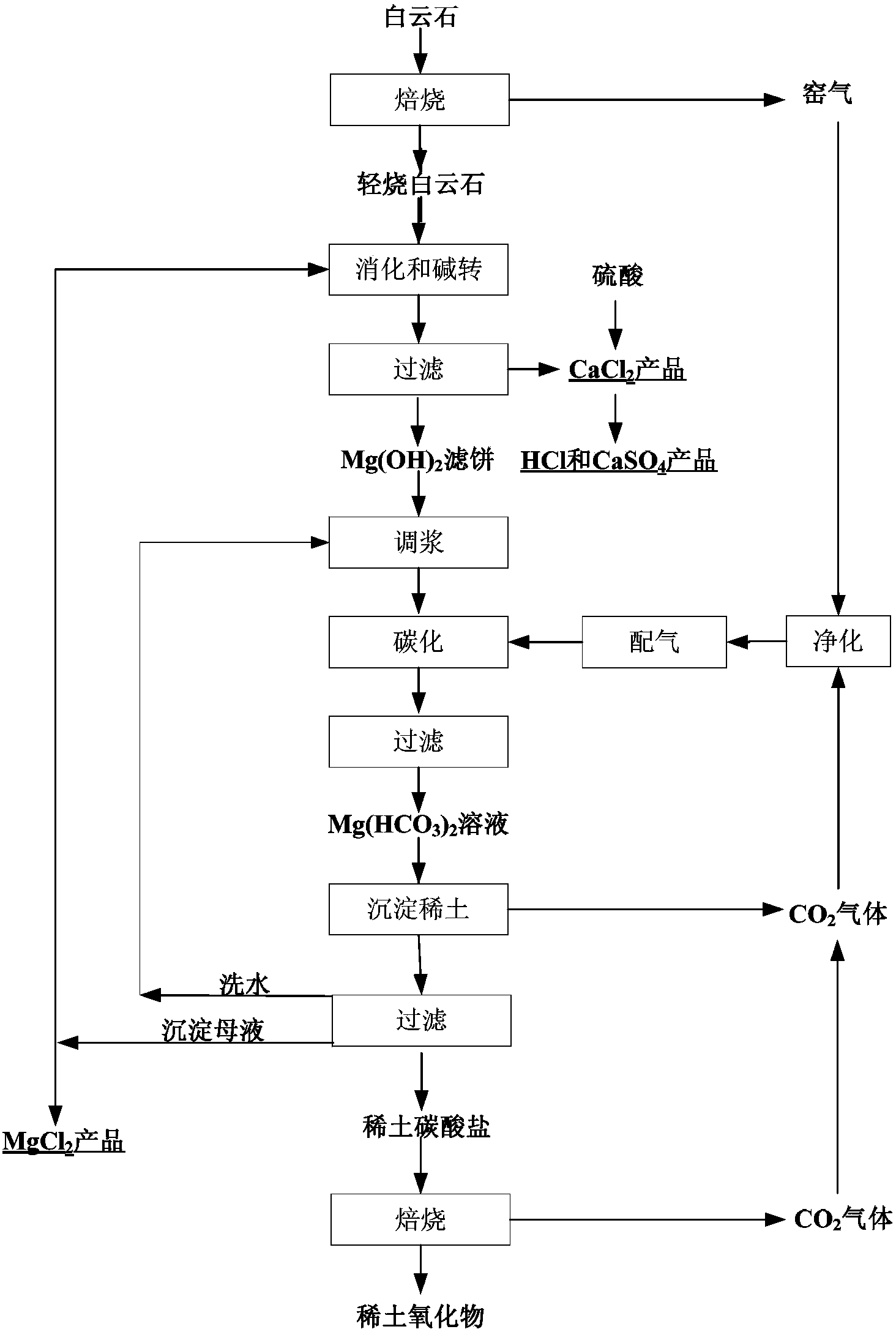
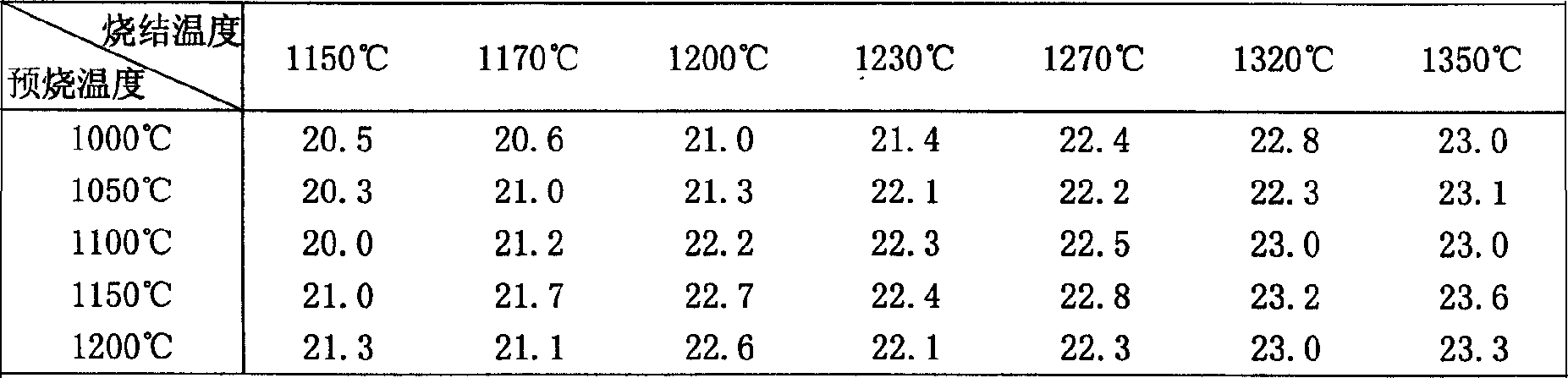
Preparation and comprehensive utilization method of magnesium bicarbonate solution
ActiveCN103382034ARealize comprehensive utilizationAchieve emissionsChlorine/hydrogen-chlorideCalcium/strontium/barium chloridesCarbonizationSlurry
The invention relates to a preparation and comprehensive utilization method of a magnesium bicarbonate solution. The method is used to prepare the magnesium bicarbonate solution used for deposition, crystallization and recovery of rare earth ions, and comprehensive recovery of calcium-compound products and magnesium-compound products by taking dolomite as a raw material. The method specifically comprises: roasting dolomite to obtain a powder containing calcium oxide and magnesium oxide, directly mixing with a magnesium chloride solution for digestion and alkali transformation at the same time to prepare a magnesium hydroxide secondary-product and a calcium compound secondary-product, and further to realize effective separation of calcium and magnesium; performing slurry mixing on magnesium hydroxide, introducing carbon dioxide gas for a carbonization reaction to prepare the magnesium bicarbonate solution; applying obtained magnesium bicarbonate to deposition and crystallization of the rare earth ions to produce rare-earth carbonate or rare-earth oxide products; and recycling one part of the filtrate to prepare magnesium bicarbonate, and evaporating one part of the filtrate for crystallization to produce the magnesium compound product.
Owner:GRIREM ADVANCED MATERIALS CO LTD
Method for modifying aluminum hydroxide/magnesium hydroxide flame retardant
ActiveCN101974257AImprove conversion rateEliminate liquid layerPigment treatment with macromolecular organic compoundsAluminium hydroxideRoom temperature
The invention relates to a method for modifying aluminum hydroxide / magnesium hydroxide flame retardant. A free-radical polymerization monomer is adsorbed on the powder surface to form apparently dry powder; the dry powder is irradiated in a radiation field so as to induce the free-radical polymerization of the monomer; then the mixture is left to stand in room temperature to finish the free-radical polymerization; and a uniform dense polymer enwrapping film is formed on the powder surface, and the polymer film-enwrapped modified modifying aluminum hydroxide / magnesium hydroxide flame retardant is obtained. The method has the advantages of low energy consumption, no pollution, high yield, simple processing and the like; and the product has the advantages of high flame retardance, tensile strength, breaking elongation strength, mechanical processing performance and the like.
Owner:广东宇星阻燃新材股份有限公司
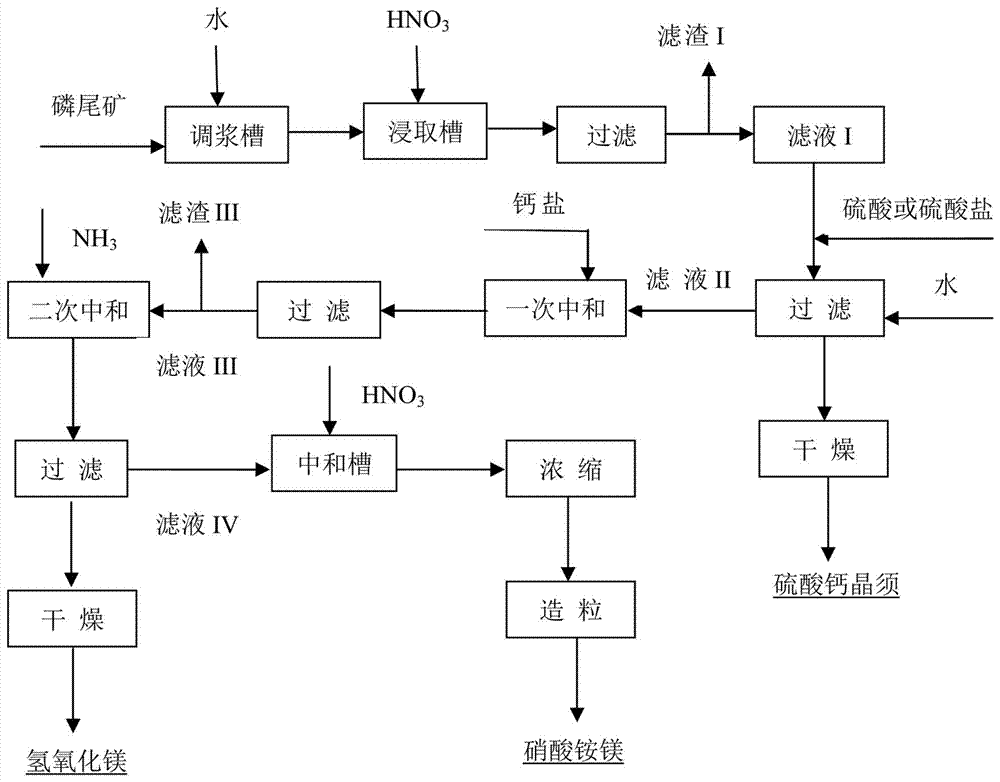
Method for preparing calcium sulfate whiskers and magnesium hydroxide by decomposing phosphate tailings through hydrochloric acid
InactiveCN107098372ASolve unhandled problemsMitigating the security concerns of stockpilingMagnesium chloridesCalcium/strontium/barium sulfatesSlurryToxic industrial waste
The invention relates to a method for preparing calcium sulfate whiskers and magnesium hydroxide by decomposing phosphate tailings through hydrochloric acid. The method comprises the following steps: adding phosphate tailings and hydrochloric acid into a decomposing tank to adequately react in the decomposing tank, and carrying out solid-liquid separation I on reacted slurry, so as to separate first mother solution and solid waste residues, wherein the solid waste residues are acid-insoluble substances; adding sulfuric acid into the first mother solution to react for crystallization, so as to generate the calcium sulfate whiskers; carrying out solid-liquid separation on crystallized slurry, so as to separate calcium sulfate dihydrate whiskers, wherein the mother solution is a mixed mother solution containing magnesium chloride and hydrochloric acid; after magnesium chloride in the mixed mother solution reaches a certain concentration, starting to take out a part of mixed mother solution, carrying out flash evaporation to evaporate HCl in the mother solution so as to obtain the mother solution mainly containing magnesium chloride, and returning the rest part of the mixed mother solution to the decomposing tank; introducing ammonia gas into the magnesium chloride mother solution, and carrying out solid-liquid separation, so as to obtain a relatively pure magnesium chloride mother solution and magnesium ammonium chloride solids containing a small amount of impurities; and absorbing HCl gas in the front stage by a magnesium ammonium chloride solution to enable the pH value of the magnesium ammonium chloride solution to be close to neutrality, and carrying out concentration and crystallization, so as to obtain magnesium ammonium chloride crystals. The method has the advantage that magnesium hydroxide and the calcium sulfate whiskers which have relatively high quality can be produced from industrial waste and the cheap raw materials.
Owner:HUBEI SANNING CHEM
Integrated circulating fluidized bed for sewage nitrogen and phosphorus recycling
InactiveCN106512465APromote growthSave dosing costSolution crystallizationProduct crystals bed crystallizationCompound (substance)Cistern
The invention provides an integrated circulating fluidized bed for sewage nitrogen and phosphorus recycling. The fluidized bed reactor for sewage nitrogen and phosphorus recycling is mainly composed of a fluidized bed, a cylindrical overflow water outlet area, a circulating water pump, a water distribution box, a rotor flow meter, circulating pipelines and valves; the fluidized bed sequentially comprises a conical crystallization area and a cylindrical crystallization and precipitation area from bottom to top, the bottom of the water distribution box is connected with the circulating water pump through a pipeline, and the rotor flow meter is arranged on a connecting pipe section; a water outlet of the circulating water pump is connected with the cone tip of a cone arranged at the bottom of the fluidized bed; the top end of the cylindrical crystallization and precipitation area is connected with a catchment cofferdam, and pores are formed in the bottom of the cofferdam to be connected with a water outlet pipe section and a return pipe section; a return pipe is connected into the water distribution box from the upper end of the water distribution box; a chemical putting system is composed of a basic magnesium carbonate dissolving tank, an ammonium chloride dissolving tank, a metering pump and a chemical adding pipeline and is connected with the lower portion of the conical crystallization area.
Owner:LANZHOU JIAOTONG UNIV
Process method for preparing magnesium hydroxide from magnesium sulfate
InactiveCN103950957AHigh yieldIncrease productionMagnesiaMagnesium hydroxideSolubilityResource utilization
The invention relates to a method for preparing magnesium hydroxide from magnesium sulfate. The method comprises the steps of separating out gypsum by precipitation reaction of a magnesium sulfate solution and a calcium chloride solution to obtain a magnesium chloride solution; further performing calcium-magnesium separation reaction on the magnesium chloride solution and dolomite milk to enable calcium hydroxide in dolomite milk to form the calcium chloride solution with high solubility to enter a liquid phase and enable magnesium hydroxide in dolomite milk to enter a solid phase, washing and drying a solid-phase matter, namely magnesium hydroxide after solid-liquid separation to obtain magnesium hydroxide or calcining to obtain magnesium oxide; returning a liquid-phase matter, namely calcium chloride liquid to perform circulating reaction process with the magnesium sulfate solution. The method is particularly suitable for treatment of a flue gas desulfurization product, namely magnesium sulfate to realize resource utilization and reutilization and realize high-added value economic benefits.
Owner:彭振超 +1
Method for preparing alkaline type magnesium carbonate by low temperature pyrogenation of Mg(HCO3)2 water and coproducing magnesium silicate
InactiveCN1970451AHigh yieldLow pyrolysis temperatureMagnesium silicatesMagnesium carbonatesMagnesium bicarbonateSodium silicate
The invention discloses a recycling method of magnesium in the heavy magnesium water solution, which comprises the following steps: aerating air in the heat decomposing reactor bottom under indoor temperature; reducing partial pressure of carbon dioxide through extracting into vacuum; transmitting 80-90% magnesium bicarbonate in the heavy magnesium solution into the sediment of basic magnesium carbonate sediment; filtering; drying to obtain the basic magnesium carbonate; adding sodium silicate in the filtrate; reacting residual 10-20% magnesium bicarbonate and sodium silicate to obtain magnesium silicate; recycling magnesium in the heavy magnesium solution completely.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
Ceramic materials for microwave ceramic capacitor and making method therefor
InactiveCN1690014AExcellent microwave dielectric propertiesRaw materials are cheapFixed capacitor dielectricCeramicsMicrowave dielectric propertiesTitanium dioxide
The ceramic material for microwave ceramic capacitor has the composition of MgTiO3-CaTiO3, and may be expressed in xMgTiO3-(1-x)CaTiO3, with x being 0.9-1.0. The preparation process includes: mixing basic magnesium carbonate, calcium carbonate and diboron pentoxide in certain proportion, ball milling, stoving, sieving and pre-sintering to obtain fused block; secondary ball milling, stoving, pelletizing, pressing to form and sintering to obtain the ceramic material. The ceramic material for microwave ceramic capacitor has excellent microwave dielectric performance and low cost.
Owner:TIANJIN UNIV
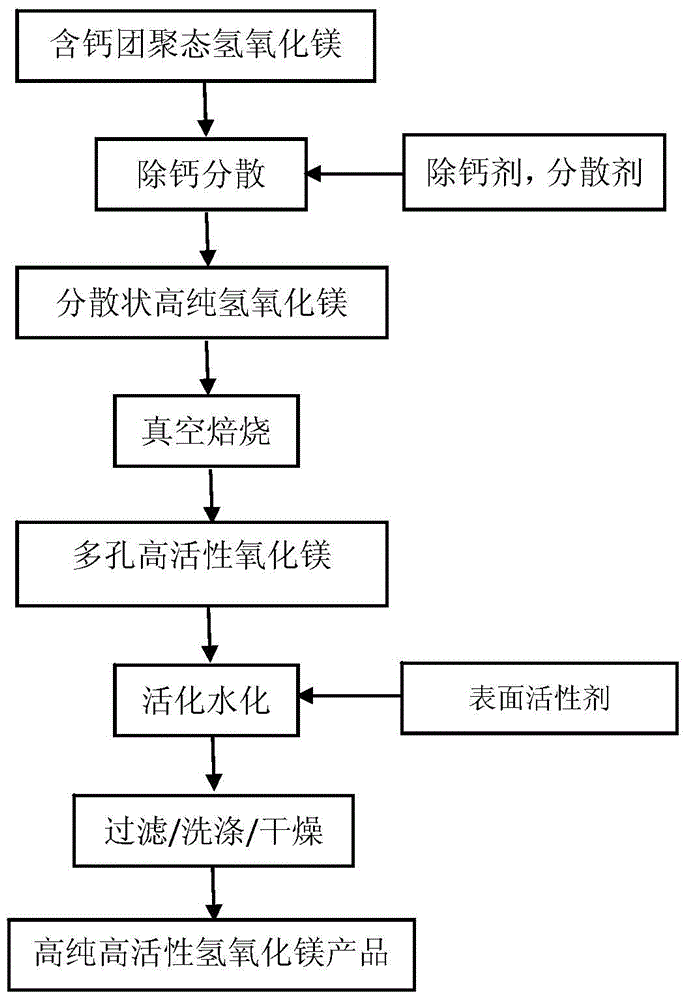
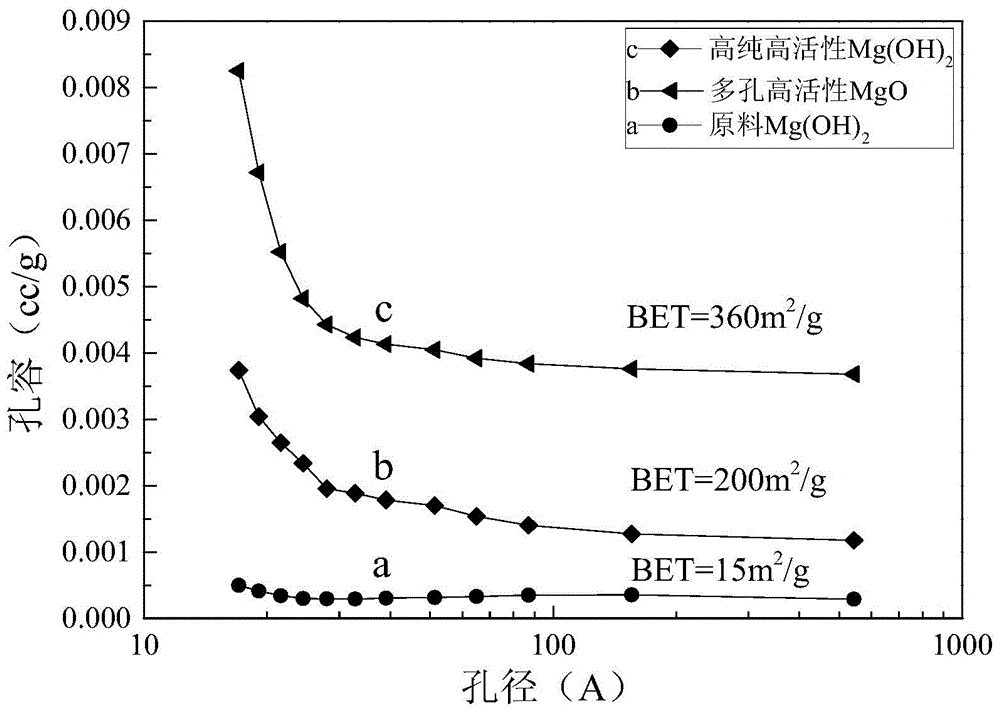
Method for preparing high-purity high-activity magnesium hydroxide through calcium-containing magnesium hydroxide
ActiveCN104891534AInhibition of recrystallizationAvoid hole fusionMagnesium hydroxideInorganic ChemicalSorbent
The invention provides a method for preparing high-purity high-activity magnesium hydroxide through calcium-containing magnesium hydroxide and belongs to the technical field of preparation of inorganic chemical materials. The method comprises the following steps: by using calcium-containing magnesium hydroxide as a raw material, firstly, carrying out decalcification and dispersion on the raw material through a liquid phase conversion method by using a calcium remover and a dispersing agent so as to obtain dispersed high-purity magnesium hydroxide; carrying out activated roasting on magnesium hydroxide at a low-medium temperature vacuum condition, so as to obtain high-specific-surface-area porous magnesium oxide; adding magnesium oxide in an aqueous solution containing a surfactant for activation conversion so as to obtain a high-purity high-activity magnesium hydroxide product. According to the method, the process is simple, the process is mild and easy to control, the product purity is high, the specific surface area is large, the activity is good and the high-purity high-activity magnesium hydroxide can be used as a basic inorganic chemical product or an effective adsorbent to be applied to the fields of medicine, inflaming retarding, metallurgy, catalyzing, desulfurization, adsorption and the like.
Owner:TSINGHUA UNIV
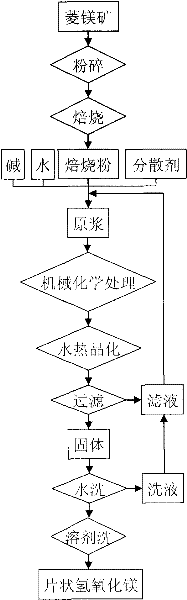
Method for preparing submicron flaky magnesium hydroxide from magnesite
The invention provides a method for preparing submicron flaky magnesium hydroxide from magnesite. The method comprises the following steps: 1) roasting smashed magnesite so as to obtain roasted magnesite powder; 2) mixing the roasted magnesite powder, alkali, a dispersing agent and water at a weight ratio of 1: (0.1-20): (0.001-0.1): (1-50) so as to obtain slurry; 3) carrying out mechanochemistry processing on the slurry in the step 2 so as to obtain the slurry to be crystallized; 4) placing the slurry to be crystallized from the step 3) in a reaction kettle, and carrying out self-press hydrothermal crystallization at the temperature of 60-250 DEG C; and 5) carrying out solid-liquid separation, washing and drying on the slurry crystallized in the step 4 so as to obtain the submicron flaky magnesium hydroxide powder. In the invention, the submicron flaky magnesium hydroxide is prepared by using the method, thereby reducing production cost; chemical salt byproducts are not generated by the process, thus the method is a clean production process; and the submicron flaky magnesium hydroxide is widely used in the fields of inflaming retardant materials, catalytic carriers and other functional materials.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Method for preparing magnesium hydroxide, magnesium and magnesium aluminate spinel by bischofite
InactiveCN102817041AHigh priceLow impurity contentElectrolysis componentsProcess efficiency improvementChemical industryElectrolysis
The invention discloses a method for preparing magnesium hydroxide, magnesium and magnesium aluminate spinel by bischofite and belongs to the field of magnesium metallurgy and chemical industry. The method comprises the steps of: taking bischofite as a raw material to produce magnesium hydroxide, hydrogen gas and chlorine gas through electrolysis, and then taking magnesium oxide, which is obtained by calcining magnesium hydroxide, as a raw material to produce magnesium metal and magnesium aluminate spinel through vacuum thermal reduction. The by-product, i.e., bischofite, during extraction of potassium salt and lithium salt in west salt lakes and in the salt manufacturing industry of the eastern coastal regions, is utilized for producing the magnesium hydroxide, magnesium oxide and magnesium aluminate spinel, therefore, the purpose of changing waste into valuable is realized. Compared with the traditional electrolysis technology of fused magnesium chloride, the method has the characteristics of low energy consumption, low cost, small environment pollution and the like.
Owner:NORTHEASTERN UNIV
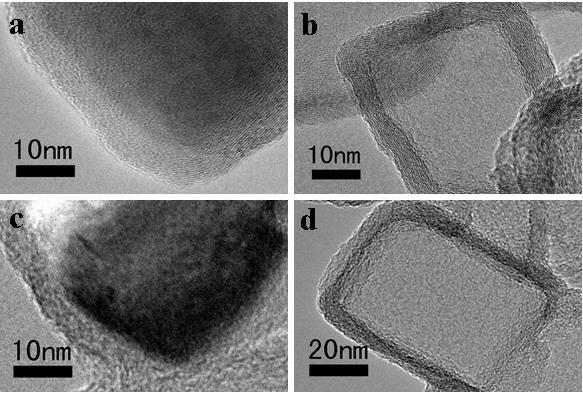
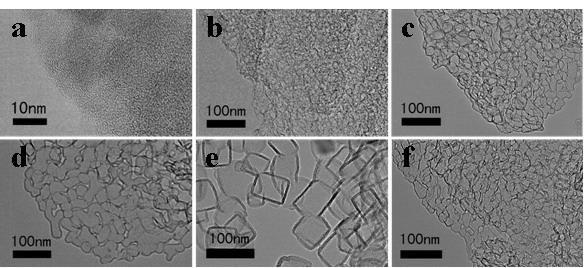
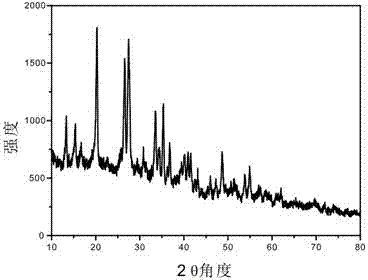
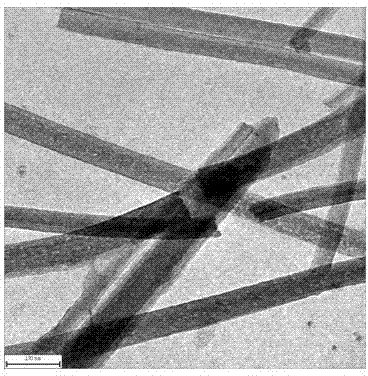
Preparation method of one-dimensional basic magnesium carbonate nano wire and porous magnesium oxide nano wire
InactiveCN102897805AHigh crystallinityHigh purityMaterial nanotechnologyMagnesium carbonatesAir atmosphereNanowire
The invention relates to a preparation method of porous magnesium oxide nano wire, belonging to the technical field of preparation of nano materials. The method comprises steps of sealing a mixed aqueous solution of metal magnesium salts and urea, heating to 90 to 130 DEG C, and reacting for 6 to 24 hours to obtain the one-dimensional basic magnesium carbonate nano wire with a diameter of 50 to 250 nanometers and a length of 10 to 500 micrometers; and then drying the basic magnesium carbonate nano wire, and calcining under an air atmosphere or an inert atmosphere to obtain the one-dimensional porous magnesium oxide nano wire. The method is simple to process and low in production cost, and is very suitable for industrial production.
Owner:ANHUI UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
Method for preparing magnesium hydroxide fire retardant with lithium carbonate by-product magnesium oxide slag
The invention relates to a method for preparing a magnesium hydroxide fire retardant with a lithium carbonate by-product magnesium oxide slag. Magnesium oxide slag is subjected to the procedures of impurity removal, grinding, screening, hydration, filtering, washing, drying and surface modification so as to obtain a magnesium hydroxide fire retardant, and waste residue generated from impurity removal can be crushed again and used as a raw material. The whole production process realizes zero discharge and reduces environmental pollution. Chemical bonding between a silane coupling agent and magnesium hydroxide generates the magnesium hydroxide fire retardant with weak surface polarity and good dispersibility. The inorganic terminal of the silane coupling agent is firstly hydrolyzed to form silanol which then reacts with the hydroxy on the filler surface. And the organic group at the other terminal of the coupling agent reacts with resin so as to form firm chemical combination. Thus, hydrophilic and oleophobic surface properties of magnesium hydroxide can be changed, the surface polarity of magnesium hydroxide is reduced, and the dispersibility as well as compatibility of magnesium hydroxide in a polymeric matrix can be improved. Therefore, the mechanical property of magnesium hydroxide in polymer product processing can be enhanced.
Owner:CHTC HELON
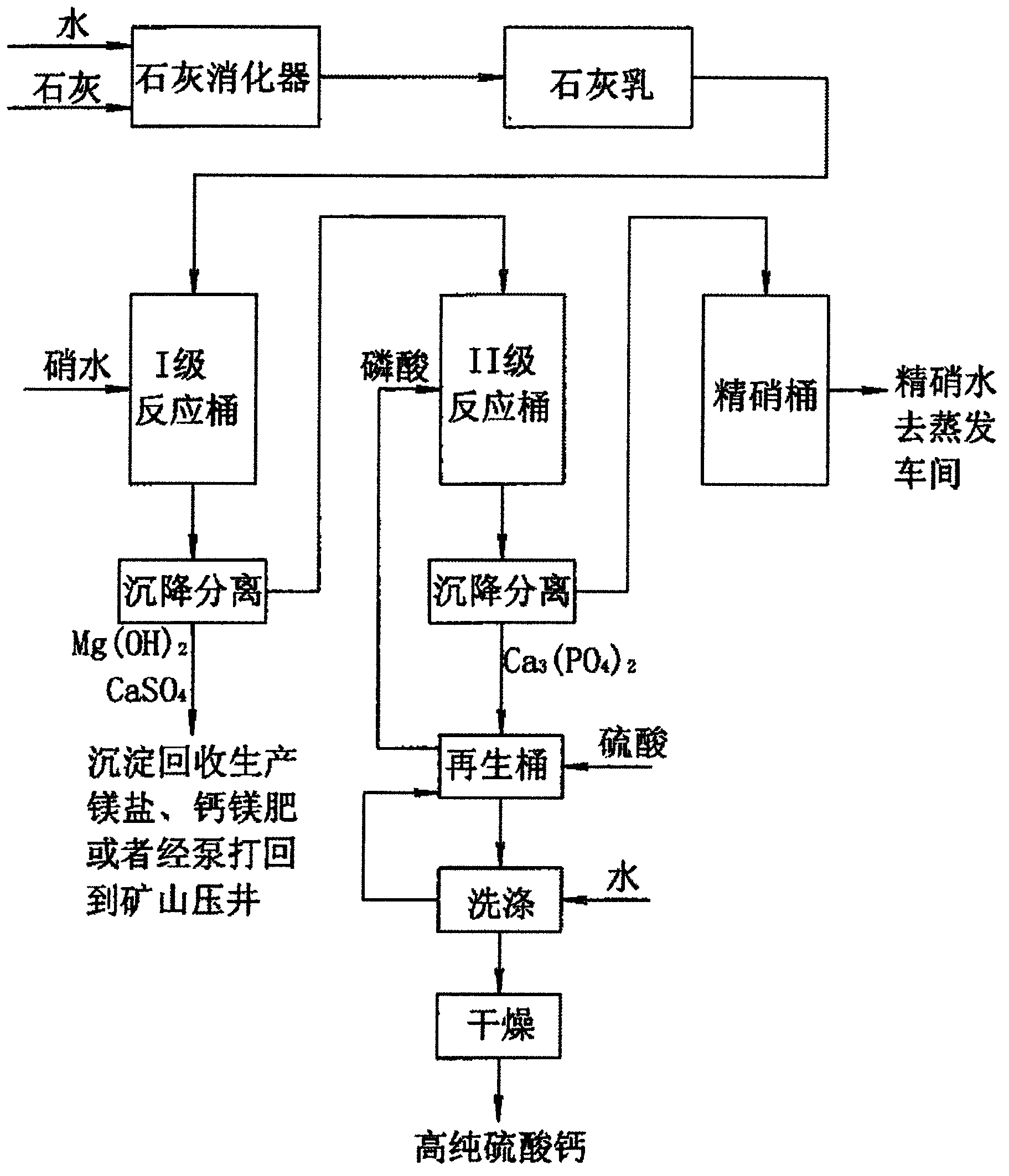
Preparation method of byproduct high-purity calcium sulphate by deep purification of nitrate aqueous solution
InactiveCN103214077AIn line with the development model of circular economyHigh purityCalcium/strontium/barium sulfatesWater/sewage treatment by flocculation/precipitationCalcium biphosphatePhosphoric acid
The invention provides a preparation method of byproduct high-purity calcium sulphate by deep purification of nitrate aqueous solution. The preparation method is characterized by comprising the following steps of: adding lime cream into nitrate aqueous solution to generate magnesium hydroxide precipitate; adding 1-5ppm of floculant, still standing the solution for 2-6 h, and carrying out settling separation; adding phosphoric acid to supernatant liquid and regulating the pH value of the solution to 9.5-12 to generate calcium phosphate precipitate; then adding 1-5ppm of floculant, still standing the solution for 2-6h, and carrying out settling separation, wherein the supernatant is pure nitrate aqueous solution and the residue is calcium phosphate; treating the residue calcium phosphate by using concentrated sulfuric acid to generate phosphoric acid and calcium sulphate and filtering by pressure to obtain the filtrate phosphoric acid for removing the next batch of material calcium ion; washing the residue by clear water to neutral state and drying the product to obtain high-purify calcium sulphate. The method disclosed in the invention adopts low-cost lime and sulphuric acid to deeply purify the nitrate aqueous solution so as to to obtain the high-purity nitrate aqueous solution and the high-purity calcium sulphate, and is simple in process and low in cost.
Owner:何京明
Lansoprazole medicinal composition tablets and preparation method thereof
ActiveCN102198109AInhibition of secretionAvoid degradationOrganic active ingredientsDigestive systemSodium bicarbonateCoating drugs
The invention discloses Lansoprazole medicinal composition tablets and a preparation method thereof. The Lansoprazole medicinal composition tablets comprise the following components in part by mass: 20 to 30 parts of Lansoprazole medicinal composition tablets, 400 to 450 parts of sodium bicarbonate, 100 to 150 parts of calcium hydrophosphate, 600 to 700 parts of magnesium hydroxide, 250 to 500 parts of filler, 40 to 50 parts of disintegrating agent, 50 to 100 parts of flavoring agent, 80 to 100 parts of bonding agent and 20 to 25 parts of lubricating agent. In the Lansoprazole medicinal composition tablets disclosed by the invention, sodium bicarbonate, calcium hydrophosphate and magnesium hydroxide are used in place of enteric coating, the secretion of gastric acid can be inhibited, the Lansoprazole can be prevented from being decomposed by gastric acid, the medicines can be absorbed quickly, and peak concentration can be reached quickly.
Owner:MITSUBISHI PHARMA GUANGZHOU
Zirconium oxide tundish pure zirconium water gap and manufacturing process thereof
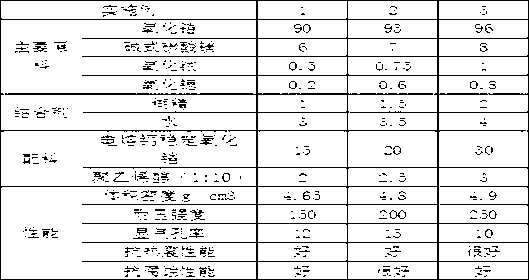
The invention discloses a zirconium oxide tundish pure zirconium water gap. The water gap is composed of a zirconium core brick and an iron cover, wherein the zirconium core brick in the tundish pure zirconium water gap is prepared from the following raw materials in parts by weight: 90-96 parts of zirconium oxide, 6-8 parts of basic magnesium carbonate, 0.5-1 part of iridium oxide, 0.2-0.8 part of strontium oxide, binding agent which consists of 1-2 parts of dextrin and 3-4 parts of water, and auxiliary materials including 15-30 parts of electrically fused calcium oxide stabilized zirconium oxide and 2-3 parts of a polypropylene with a mass ratio of 1:10. Furthermore, the invention discloses a manufacturing process of the zirconium oxide tundish pure zirconium water gap. The process disclosed by the invention can be used for enhancing the stability of the zirconium oxide, and the auxiliary materials added in melting process can guarantee moulding density and blank strength and can control product shrinkage within 7-9%; and the zirconium oxide tundish pure zirconium water gap prepared by the invention can be used for greatly improving the service life, the erosion resistance performance and thermal shock performance.
Owner:TAICANG HONGDA JUNMENG NEW MATERIAL
Method for preparing magnesium oxide or magnesium oxide and fibrous magnesium hydroxide from magnesite
InactiveCN106745103AWide variety of sourcesLow costMagnesiaMagnesium hydroxideWater bathsMagnesium salt
The invention discloses a method for preparing magnesium oxide from magnesite. The method comprises the following steps: 1) calcining magnesite to obtain caustic-burned magnesia powder; 2) adding the caustic-burned magnesia powder into an ammonium salt solution for leaching, wherein the leaching process is performed under the conditions of reduced pressure, water bath heating and stirring; and 3) filtering to obtain magnesium salt leachate; 4) enabling the magnesium salt leachate to react with ammonia water to obtain magnesium hydroxide precipitate, and calcining to obtain magnesium oxide. The invention also discloses a method for preparing magnesium oxide and fibrous magnesium hydroxide from magnesite, which comprises the following steps: 1) calcining magnesite to obtain caustic-burned magnesia powder; 2) adding the caustic-burned magnesia powder into an ammonium salt solution for leaching, wherein the leaching process is performed under the conditions of reduced pressure, water bath heating and stirring; 3) extracting upper- and middle-layer suspension of the solid-liquid mixed system and performing suction filtration to obtain filtrate and a filter cake; drying the filter cake to obtain fibrous magnesium hydroxide; and combining the precipitate slag washing liquid with the filtrate to obtain magnesium salt leachate; and 4) enabling the magnesium salt leachate to react with ammonia water to obtain magnesium hydroxide precipitate, and calcining to obtain magnesium oxide.
Owner:张旭
Basic magnesium carbonate, production method and use of the same
ActiveUS7922991B2Large specific surface areaImprove featuresCalcium/strontium/barium carbonatesBiocideCarbonateTubular aggregates
Owner:NITTETABU MINING CORP
Method for preparing calcium sulfate whisker by-product magnesium hydroxide and magnesium ammonium nitrate by decomposing phosphate tailings by nitric acid
InactiveCN107879363ARealize resource utilizationImprove economyPolycrystalline material growthMagnesium hydroxidePhosphateSlurry
The invention discloses a method for preparing calcium sulfate whisker by-product magnesium hydroxide and magnesium ammonium nitrate by decomposing phosphate tailings by nitric acid. The method comprises the following steps: 1) adding water into phosphate tailings to prepare phosphate tailing pulp, adding a nitric acid solution, carrying out an acidolysis reaction, and filtering reaction slurry toobtain a mother liquor I and a filter residue I; 2) adding sulfuric acid or a sulfate into the mother solution I, carrying out a reaction, then carrying out cooling crystallizing, and carrying out filtering separation to obtain a filter cake II and a mother liquor II, washing and drying the filter cake II to obtain calcium sulfate whiskers, and bringing the mother liquor II back to the step 1) for an acidolysis reaction; 3) adding a calcium salt into a mother solution II subjected to multiple times of reaction, carrying out a reaction, and carrying out filtering separation to obtain a filterresidue III and a mother liquor III; and 4) introducing ammonia into the mother liquor III, carrying out a reaction, carrying out filtering separation to obtain a filter cake IV and a mother liquor IV, washing and drying the filter cake IV to obtain a magnesium hydroxide finished product, adding nitric acid into the mother liquor IV, carrying out a reaction, and carrying out concentration and granulation on the mother liquor IV to obtain the byproduct magnesium ammonium nitrate. According to the invention, comprehensive utilization of phosphorus tailing resources is realized, waste is changedinto wealth, and the green chemical concept is conformed.
Owner:HUBEI SANNING CHEM
Simple nanometer magnesium oxide preparation method
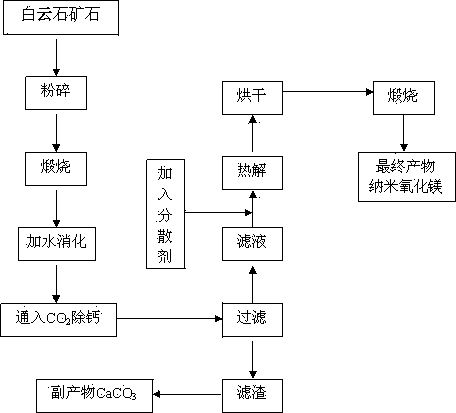
The present invention relates to a simple nanometer magnesium oxide preparation method, which comprises: crushing dolomite, calcining at a temperature of 950-1200 DEG C, digesting the product at a temperature of 50-90 DEG C after calcining, introducing CO2 to the digested product at a room temperature to carry out a carbonization reaction, stopping CO2 introduction when the pH value of the system is 7-9, carrying out solid-liquid separation on the reaction product to obtain a magnesium bicarbonate solution (Mg(HCO3)2 solution), adding a dispersant to the magnesium bicarbonate solution according to a certain ratio, completely and uniformly stirring for 20-60 min, carrying out pyrolysis for 30-90 min at a temperature of 80-100 DEG C until the solid basic magnesium carbonate is precipitated, and calcining the solid basic magnesium carbonate for 30-60 min at a temperature of 450-750 DEG C to obtain the nanometer magnesium oxide with an average particle size of 50 nm. According to the present invention, the used raw materials are abundant, the price is low, the process is simple, characteristics of safety and no pollution are provided, the process is easy to control, the nanometer magnesium oxide prepared by using the method has a characteristic of uniform particle size, and the method is suitable for industrial development of nanometer magnesium oxide production.
Owner:CHANGCHUN UNIV OF TECH
Method for producing light calcium carbonate and magnesium sulfate heptahydrate from salty mud
ActiveCN109574055AIncrease the solid-to-liquid ratioIncrease the carbonization reaction rateCalcium/strontium/barium carbonatesMagnesium sulfatesMagnesium saltCarbonization
The invention provides a method for producing light calcium carbonate and magnesium sulfate heptahydrate from salty mud. The method includes mixing the salt mud (mainly comprising magnesium hydroxideand calcium carbonate) produced by a brine refining process with calcium sulfate, adding a certain amount of water for pulping, injecting carbon dioxide for a carbonization reaction to convert the calcium sulfate and the magnesium hydroxide into calcium carbonate sediments and soluble magnesium sulfate, and performing filtration to separate calcium and magnesium salt; drying a filter cake to obtain a light calcium carbonate product, and concentrating, crystalizing and drying filtrate to obtain a magnesium sulfate heptahydrate product. The method has the advantages that the greenhouse gas carbon dioxide is fastened while the light calcium carbonate and magnesium sulfate heptahydrate products are obtained through compressive utilization of the calcium and magnesium salt in the salty mud, harmless comprehensive utilization of the salty mud is realized, and certain economic benefits can be acquired.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
Method for preparing sodium metaborate with coproduct of light magnesium carbonate from low-grade ores containing magnesium and boron
InactiveCN104386705ARealize cleaner productionReduce alkalinityMagnesium carbonatesBoratesDecompositionLower grade
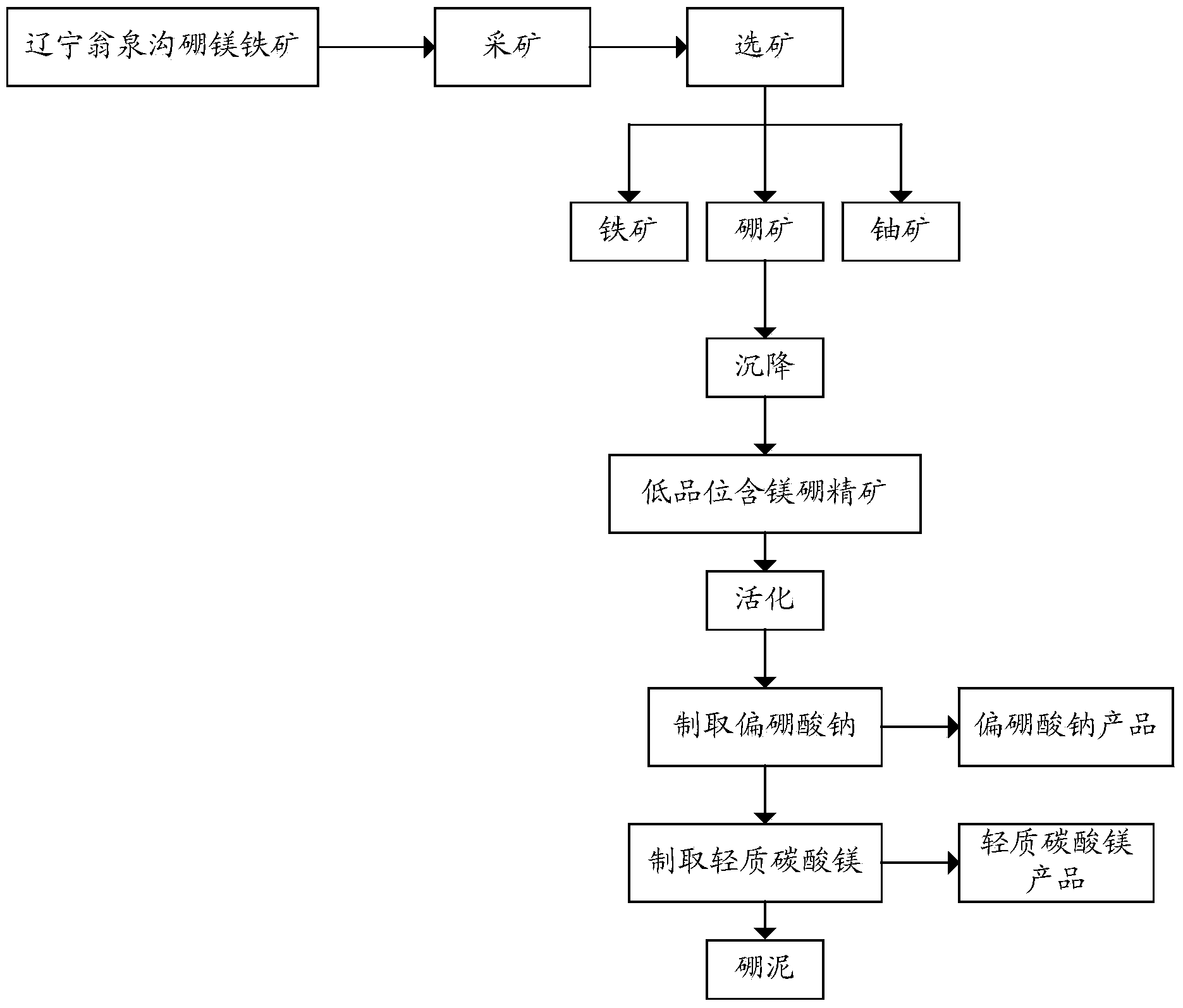
The invention provides a method for preparing sodium metaborate with a coproduct of light magnesium carbonate from low-grade ores containing magnesium and boron. The method comprises the following steps: activating the ores containing magnesium and boron to obtain an activated ore concentrate containing magnesium and boron; performing sodium modification on the activated ore concentrate containing magnesium and boron to obtain a sodium metaborate solution and alkaline hydrolysis boron mud; performing concentration crystallization on the sodium metaborate solution to obtain hydrated sodium metaborate; mixing the alkaline hydrolysis boron mud with water to obtain an alkaline hydrolysis boron mud mixture, and carbonizing the alkaline hydrolysis boron mud mixture by adopting a carbon decomposition gas to obtain a heavy magnesium solution and boron mud; and pyrolyzing the heavy magnesium solution to obtain light magnesium carbonate. According to the method, the low-grade ore concentrate containing magnesium and boron is activated at first so as to improve the activity of boron and ensure that borne can be fully extracted; meanwhile, ores containing magnesium are decomposed to generate magnesium hydroxide, which creates a condition for subsequent extraction of magnesium compounds; and by virtue of sodium modification and carbon decomposition, the extraction of boron and magnesium from the activated ore concentrate containing magnesium and boron is realized.
Owner:LIAONING SHOUGANG BORON IRON
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com