Preparation method of byproduct high-purity calcium sulphate by deep purification of nitrate aqueous solution
A deep purification, calcium sulfate technology, applied in the direction of calcium/strontium/barium sulfate, flocculation/precipitation water/sewage treatment, etc., can solve the problems of reducing the flow of nitrate water, perforation, rising energy consumption, etc., to achieve good purification effect, Simple process and low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
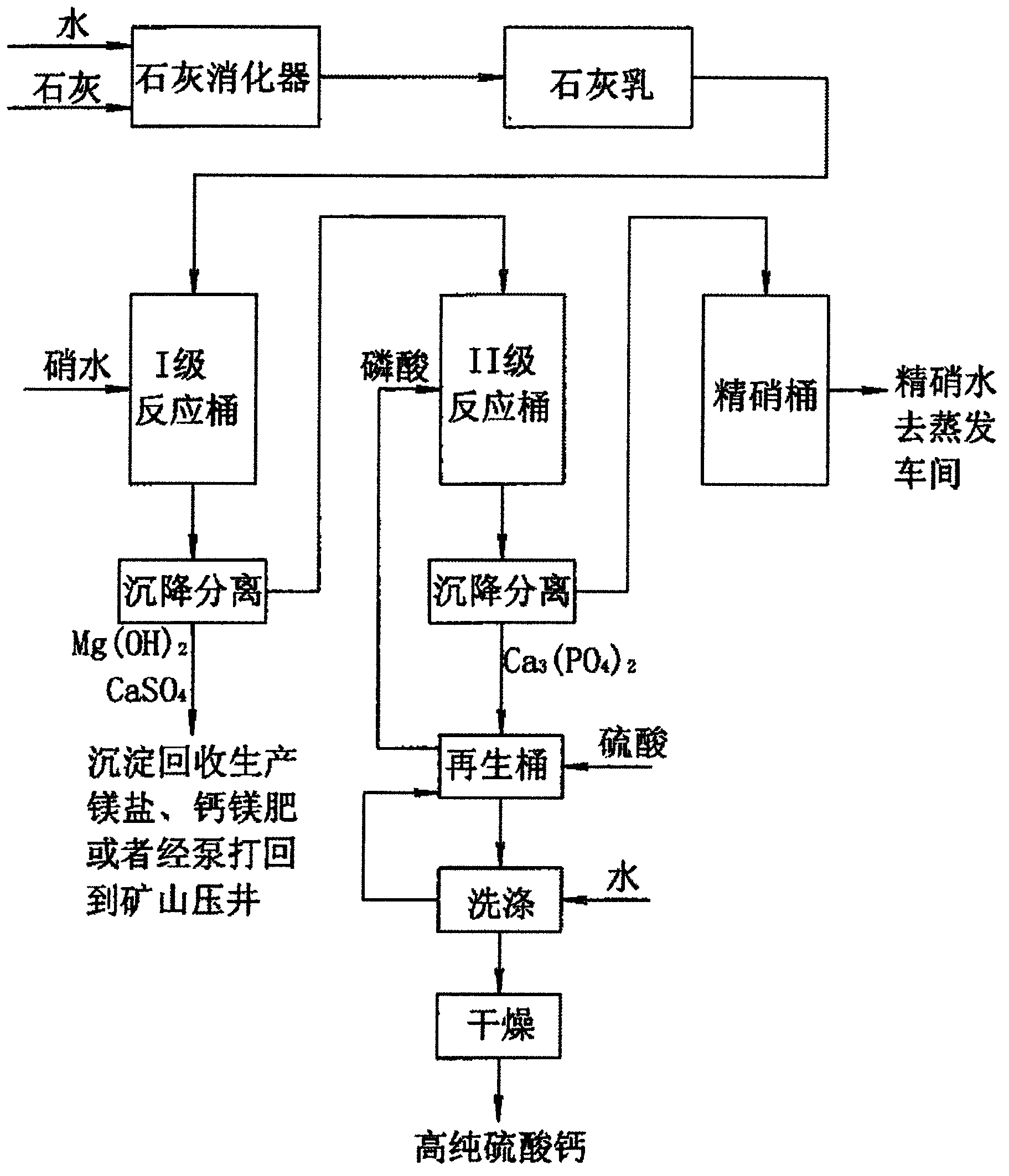
[0028] Take 10 liters of nitric water, which contains Mg 2+ 0.32g / L, Ca 2+ 0.69g / L, Na 2 SO 4 220g / L, Cl - 7g / L. When purifying and treating nitric water, first prepare lime emulsion: take 50g of quicklime and put it into a lime digester, add 300g of water, stir evenly, and obtain lime emulsion.
[0029] Add lime emulsion, calcium hydroxide and magnesium ions (Mg 2+ ) reacts to generate magnesium hydroxide to remove magnesium ions, and excess calcium hydroxide removes Na 2 SO 4 Causticize into NaOH, when the generated NaOH content reaches 1.5g / L, stop adding lime emulsion; continue to react at room temperature for 0.5h, add flocculant sodium polyacrylate, the usage amount is 1ppm, precipitate and clarify for 6h, filter; add phosphoric acid (about 13g of 80% phosphoric acid), adjust the pH value to 9.5; stir and react at room temperature for about 1h, add 1ppm flocculant sodium polyacrylate, let stand for 6h, filter, and the filtrate is refined nitric water. After testin...
Embodiment 2
[0032] Take 10 liters of brine, which contains Mg 2+ 0.40g / L, Ca 2+ 0.76g / L, Na 2 SO 4 20g / L, NaCl297g / L. When purifying brine, first prepare lime emulsion: take 100g of quicklime and put it into a lime digester, add 500g of water, stir evenly to obtain lime emulsion. Add lime emulsion under stirring of brine, excess calcium hydroxide 2 SO 4 Causticize to NaOH, when the generated NaOH content reaches 2.0g / L, stop adding lime emulsion; continue to react at room temperature for 45min, add cationic polyacrylamide, the usage amount is 3ppm, precipitate and clarify for 4h, filter; add phosphoric acid (about 14g80% Phosphoric acid), adjust the pH value to 11.0; stir and react at room temperature for about 1 hour, add 3 ppm cationic polyacrylamide, let stand for 4 hours, filter, the filtrate is refined brine, after testing, the refined brine has no calcium and magnesium ions;
[0033] Add clear water to the filter residue to make a slurry with a solid content of 10-20%, add 20g...
Embodiment 3
[0035] Take 10 liters of nitric water, which contains Mg 2+ 0.36g / L, Ca 2+ 0.72g / L, Na 2 SO 4 230g / L, NaCl9g / L. When purifying and treating nitric water, first prepare lime emulsion: take 80g of quicklime and put it into a lime digester, add 500g of water, stir evenly, and obtain lime emulsion. Add lime emulsion under nitrate stirring, excess calcium hydroxide 2 SO 4 Causticize into NaOH, when the NaOH content of generation reaches 2.5g / L, stop adding lime emulsion; Continue to react at room temperature for 1h, add sodium polyacrylate, use amount is 5ppm, precipitate and clarify for 2h, filter; filtrate adds phosphoric acid (about 12g80% Phosphoric acid), adjust the pH value to 12.0; stir and react at room temperature for about 1 hour, add 5 ppm of sodium polyacrylate, let it stand for 2 hours, filter, and the filtrate is refined nitric water. After testing, the refined nitric water has no calcium and magnesium ions;
[0036] Add clear water to the filter residue to make...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com