Preparation and comprehensive utilization method of magnesium bicarbonate solution
A technology of magnesium bicarbonate and solution, applied in the direction of magnesium carbonate, chemical instruments and methods, magnesium halide, etc., can solve the problems of high production cost, low magnesium calcium carbonate, cost increase, etc., achieve good economic and social benefits, promote Crystal nucleation growth, effect of improving filtration performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
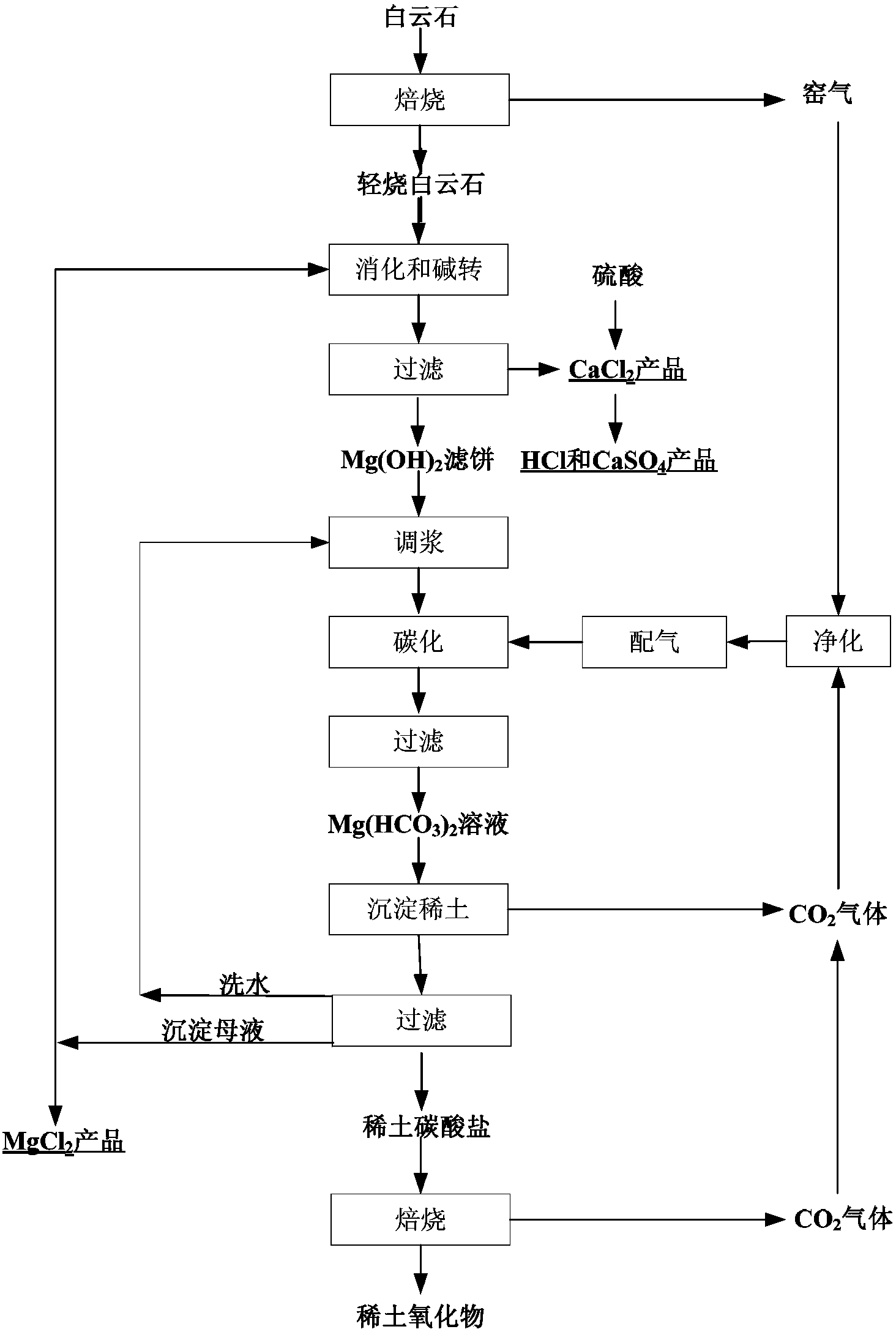
[0046] Roast the dolomite at 1000°C for 4 hours to obtain calcium oxide and magnesium oxide powder. Take 915g (CaO content of 60wt%) and add it to 5L magnesium chloride solution (2mol / L). The amount of calcium oxide in the added powder is oxidized in the magnesium chloride solution. The molar ratio of magnesium is CaO / MgO=98%, the reaction is stirred for 1 hour, the pH is 10.4, clarified and filtered, and the filtration time is 10 minutes, and then washed to obtain 2198g of magnesium hydroxide filter cake and calcium chloride filtrate. The filtrate is concentrated, Crystallize, filter, and dry to obtain 1368g of calcium chloride dihydrate by-product, with CaCl 2 The total content is 69.3%, reaching industrial-grade qualified products.
[0047] After the magnesium hydroxide filter cake is washed with calcium, the molar ratio of CaO / MgO in the filter cake is tested to be 0.8%. It is slurried with water to 12g / L (calculated as MgO), and then 80V% carbon dioxide gas is introduced into...
Embodiment 2
[0052] Roast the dolomite at 1150℃ for 4h to obtain 420kg of calcium oxide and magnesium oxide powder (CaO content is 60wt%), slowly add it to 4.2m 3 In the 1.2mol / L magnesium chloride solution, the molar ratio of the amount of calcium oxide in the powder to the amount of magnesium oxide in the magnesium chloride solution is CaO / MgO=90%, the pH of the reaction is 9.8, and then filtered and washed to obtain the magnesium hydroxide filter Cake 1262kg and calcium chloride filtrate, the filtrate is heated and concentrated to obtain 628.5kg of calcium chloride dihydrate by-product, with CaCl 2 The total content is 60.5%.
[0053] After the magnesium hydroxide filter cake is washed with calcium, the molar ratio of CaO / MgO in the filter cake is tested to be 0.1%, and the water is adjusted to 25g / L (MgO meter), and then 50V% carbon dioxide gas (kiln gas) is introduced into the magnesium hydroxide slurry. Mixed with CO produced during precipitation 2 (Gas) to a partial pressure of 0.4MPa, ...
Embodiment 3
[0056] Roast the dolomite at 950°C for 10 hours to obtain 1367g of calcium oxide and magnesium oxide powder (CaO content is 59wt%), which is mixed with 7.7L of 1.95mol / L magnesium chloride solution for digestion and alkali conversion. The amount of calcium oxide in the added powder The molar ratio CaO / MgO to the amount of magnesium oxide in the magnesium chloride solution is CaO / MgO=96%, and the pH of the reaction is 10.2, then filtered and washed to obtain 3662g of magnesium hydroxide filter cake and calcium chloride filtrate. The filtrate is heated and concentrated to obtain dihydrate chlorine Calcium by-product 2011g, with CaCl 2 The total content is 69.0%, reaching industrial-grade qualified products.
[0057] After the magnesium hydroxide filter cake was washed with calcium, the molar ratio of CaO / MgO in the filter cake was tested to be 0.5%. It was slurried with water to 9.1g / L (calculated as MgO), and then the magnesium hydroxide slurry was passed into 35V% carbon dioxide g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com