Method for preparing alkaline type magnesium carbonate by low temperature pyrogenation of Mg(HCO3)2 water and coproducing magnesium silicate
A low-temperature pyrolysis and magnesium carbonate technology, applied in the direction of magnesium silicate, magnesium carbonate, silicate, etc., can solve the problems of high energy consumption, large waste, poor management, etc., achieve low pyrolysis temperature, high decomposition rate, The effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
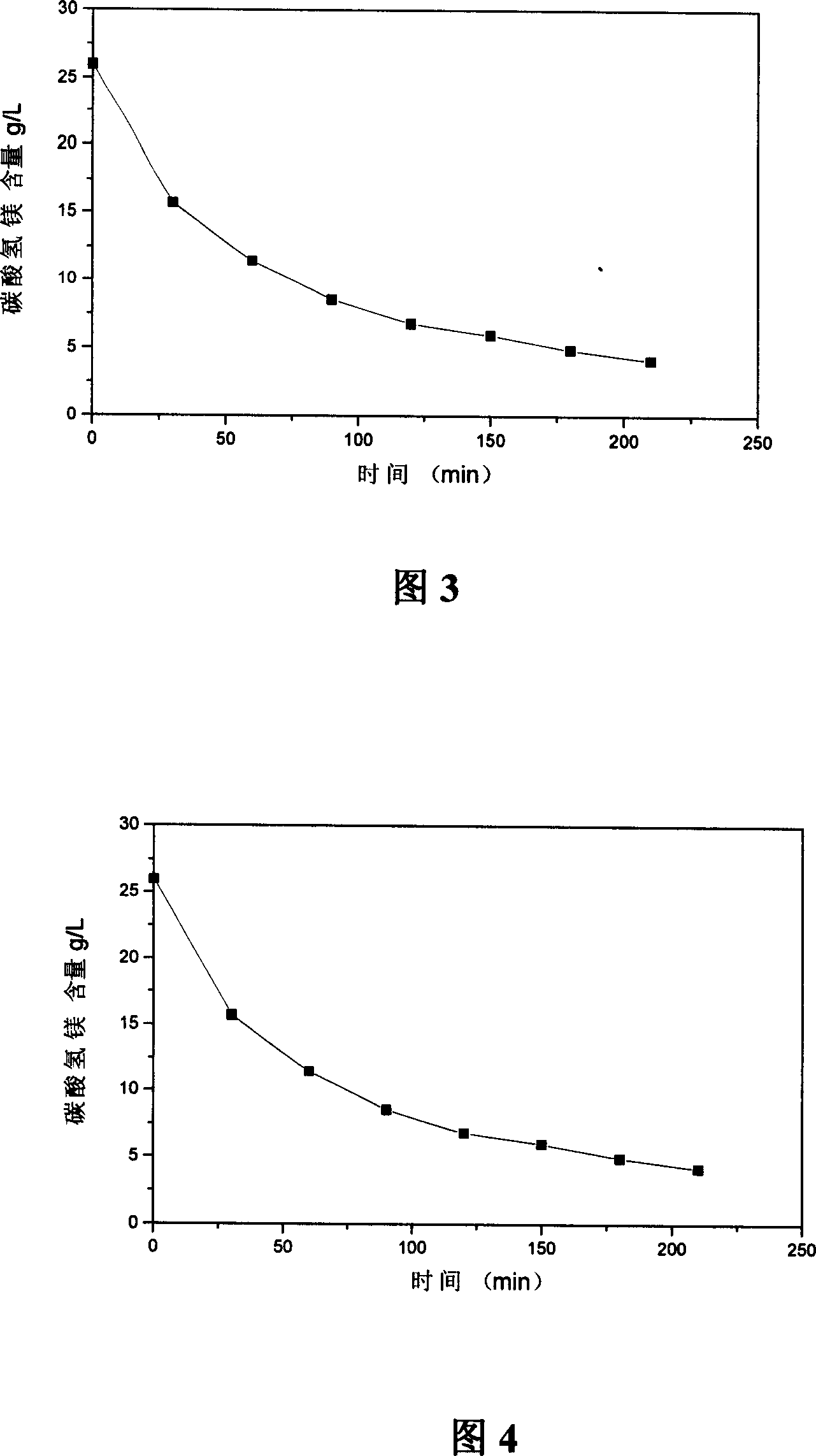
[0042] The experimental process of the present invention is shown in Figure 2. A bubbling reactor is used, the air volume is 10L / h, and it is fixed, the amount of heavy magnesium aqueous solution is 1500ml, and the gas-liquid ratio is 6.67. Experiments at 40°C and 60°C The results are shown in Figure 3 and Figure 4.
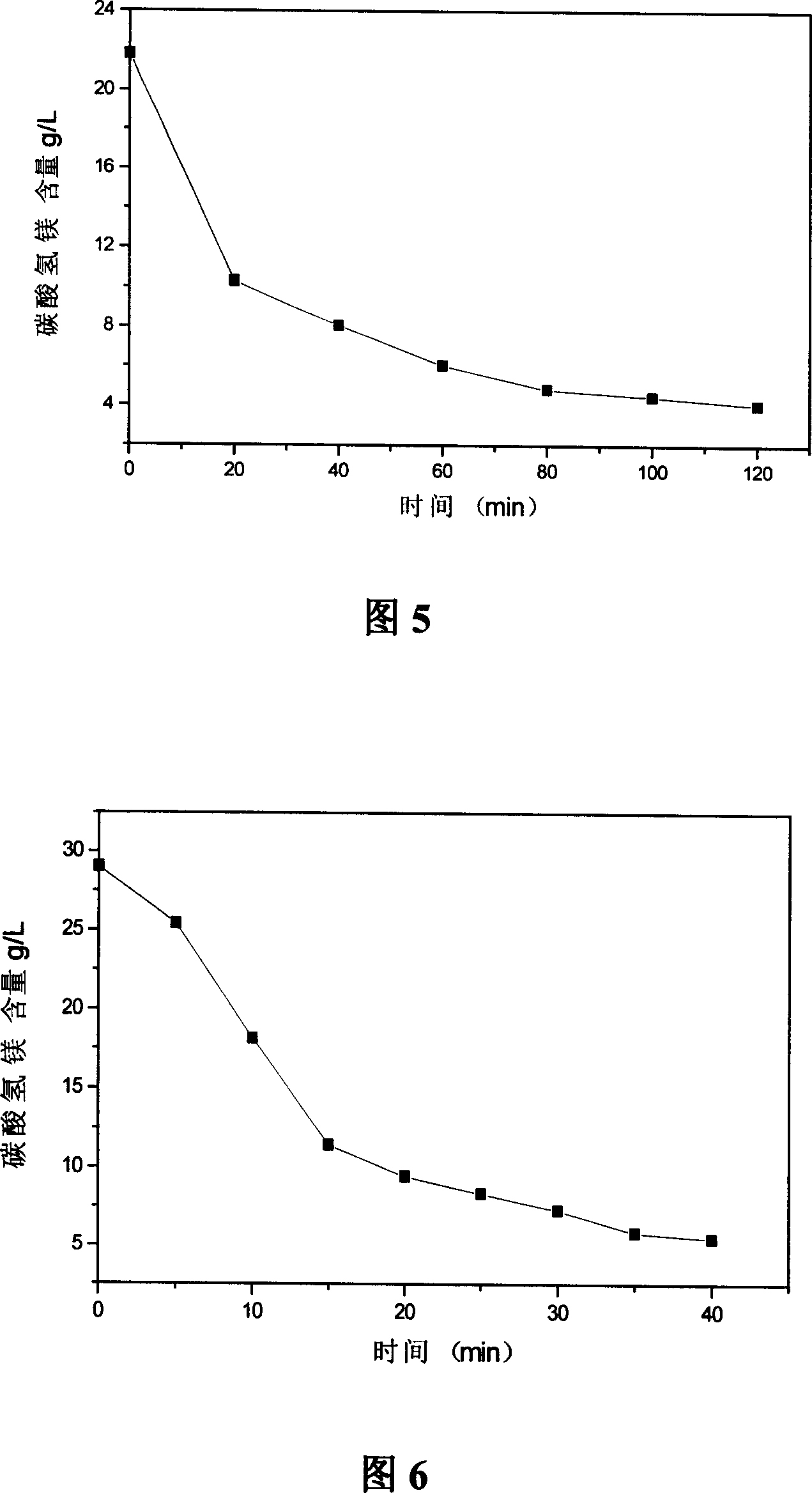
[0043] Fig. 3 is when temperature is 60 ℃, and gas-to-liquid ratio is 6.67 when the content of bicarbonate of magnesium in heavy magnesium water is the relationship diagram of time change, can find out that the decomposition rate of bicarbonate of magnesium is 85%.
[0044] Fig. 4 is when the pyrolysis temperature is 40 DEG C, the magnesium bicarbonate content changes with time in the solution graph, the decomposition rate of magnesium bicarbonate is 83.08%, and the variation with time of the two tends to be gentle after 200 minutes.
Embodiment 2
[0046]The selected air volume is 40L / h, and it is fixed, the amount of heavy magnesium aqueous solution is 1500ml, and when the gas-liquid ratio is 26.68, the experimental results of pyrolysis temperature of 40°C and time of 120 minutes are shown in Figure 5. Magnesium bicarbonate The decomposition rate is 87.1%.
Embodiment 3
[0048] Under the condition of temperature 30°C, the concentration of heavy magnesium water is 25L containing magnesium bicarbonate (29.6g / l), pumped into the spray desorption tower by centrifugal pump, the flow rate is 100L / h, and the gas flow rate is 6L / h. The results are shown in As shown in Figure 6, it can be seen that when the time is 40 minutes, the mass transfer rate is accelerated due to the liquid droplets being dispersed into the air in the form of mist droplets, the carbon dioxide desorption rate is accelerated, and the decomposition rate of light magnesium carbonate reaches 81%. .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com