Method for preparing sodium metaborate with coproduct of light magnesium carbonate from low-grade ores containing magnesium and boron
A technology of light magnesium carbonate and sodium metaborate, applied in the direction of magnesium carbonate, borate, chemical instruments and methods, etc., can solve the problems of polluting the surrounding environment, returning to alkali, and high alkali content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
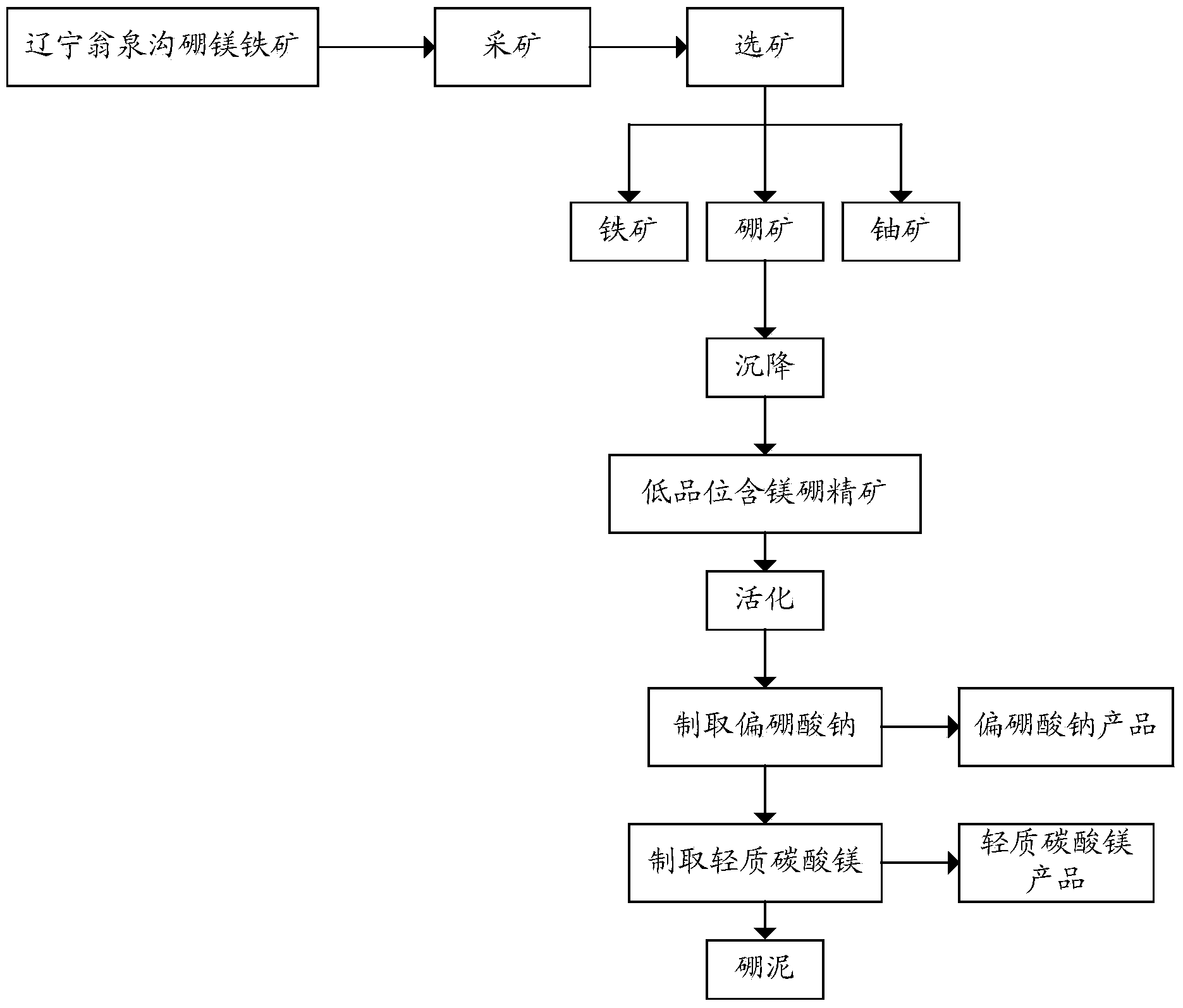
[0056] Transport the low-grade magnesium-boron-containing concentrate to the roasting kiln, and roast it at 690°C±5°C, and transport the roasted material to the alkaline hydrolysis batching tank, according to mineral powder: sodium hydroxide (calculated as solid): The mass ratio of water is 100:25:400 for batching. After the batching is completed, the mixed material is transported to the alkaline hydrolysis tank, sealed and heated to 155°C for alkaline hydrolysis extraction. After 2 hours of extraction, it begins to cool to normal pressure and 95°C. Afterwards, the obtained product is sent to the first filter for filtration to obtain alkali hydrolysis boron mud and sodium metaborate solution;
[0057] The sodium metaborate solution is concentrated and crystallized to obtain hydrated sodium metaborate crystals, which is the finished product.
[0058] The obtained alkali hydrolyzed boron mud is transported to the magnesium batching tank, and the mass ratio of alkali hydrolyzed b...
Embodiment 2
[0061]The process of preparing sodium metaborate to co-produce light magnesium carbonate is the same as that of Example 1, except that the roasting temperature in this example is 750°C±5°C.
[0062] In this embodiment, the yield of sodium metaborate prepared was 58%, the yield of light magnesium carbonate was 38%, and the pH of the finally discharged boron sludge was 8.
Embodiment 3
[0064] The process of preparing sodium metaborate to co-produce light magnesium carbonate is the same as that of Example 1, except that the concentration of sodium hydroxide in this example is 150 g / L.
[0065] In this embodiment, the yield of sodium metaborate prepared was 66%, the yield of light magnesium carbonate was 40%, and the pH of the finally discharged boron sludge was 7.7.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com