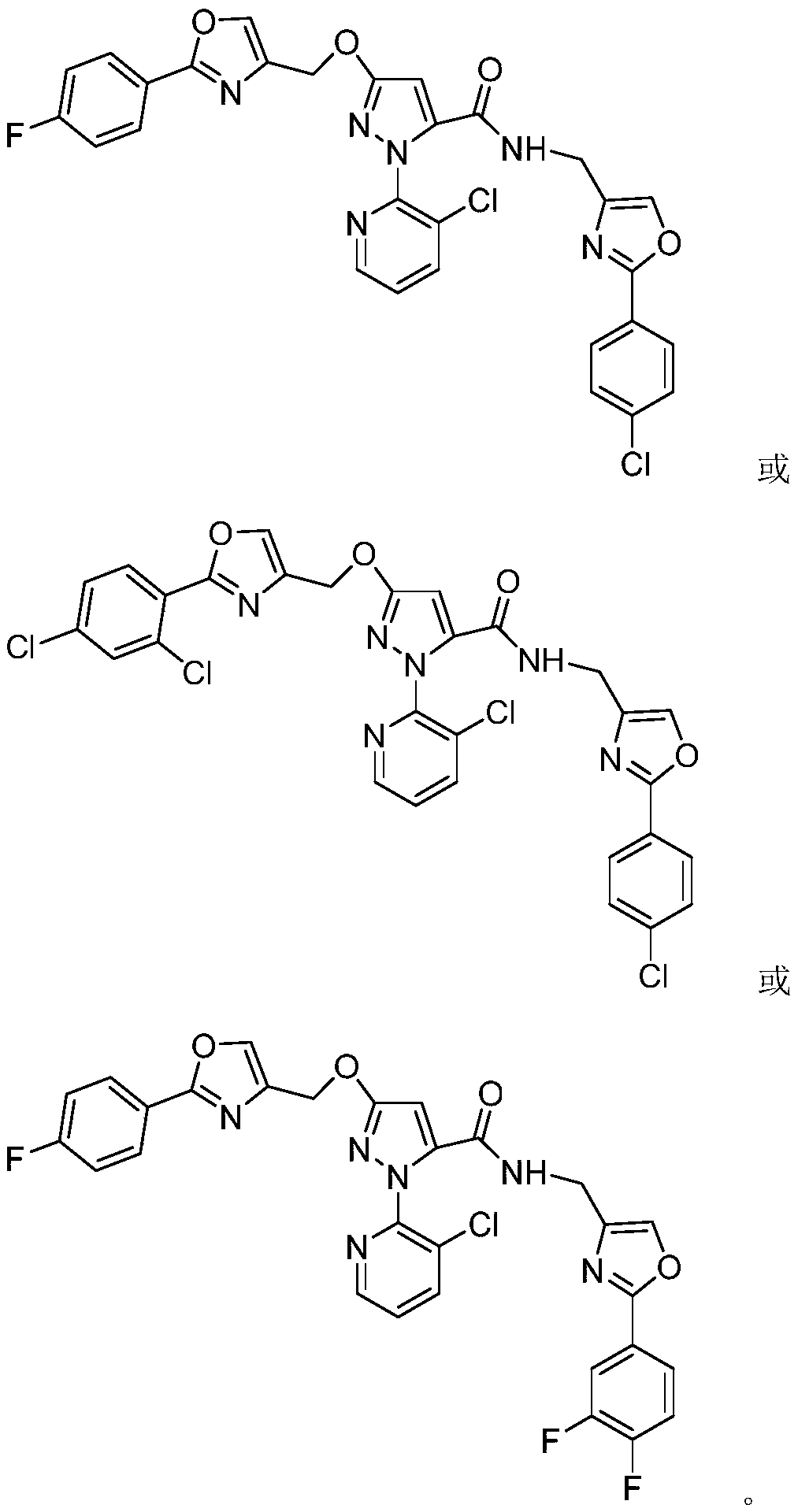
Pyrazol-5-amide derivative containing oxazole structure and preparation method and application thereof
A technology of amides and derivatives, applied in the field of pyrazole-5-amide derivatives and their preparation, to achieve excellent control effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028]
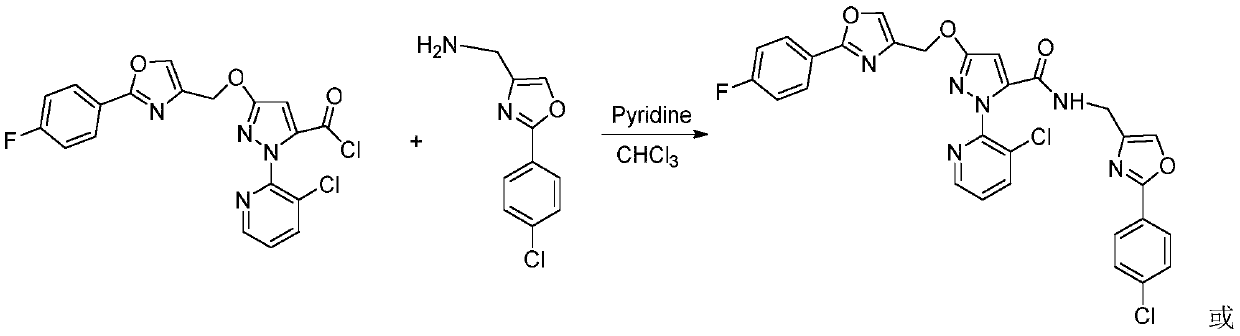
[0029] 4 mmol of compound IIIa was dissolved in 30 mL of chloroform, then 20 mmol of pyridine was added, and then 6 mmol of intermediate IIa was added thereto under ice-cooling conditions. After the addition was completed, the reaction was continued in ice bath for 9 hours. The reaction was stopped, and the reaction solution was rotary evaporated to dryness under reduced pressure, and the obtained residue was separated and purified by column chromatography to obtain the target compound Ia; 1 H NMR (400MHz, CDCl 3 ):δ8.46~8.47(m,1H,Py-H),8.03~8.08(m,2H,Ar-H),7.93(d,J=8.8Hz,2H,Ar-H),7.86~7.88( m,1H,Py-H),7.77(s,1H,Oxazole-H),7.61(s,1H,Oxazole-H),7.45(d,J=8.8Hz,2H,Ar-H),7.34-7.39 (m,1H,Py-H),7.12-7.17(m,2H,Ar-H),6.96(s,1H,NH),6.29(s,1H,Pyrazole-H),5.29(s,2H,CH 2 ), 4.45 (d, J=5.2Hz, 2H, CH 2 ).
Embodiment 2
[0031]
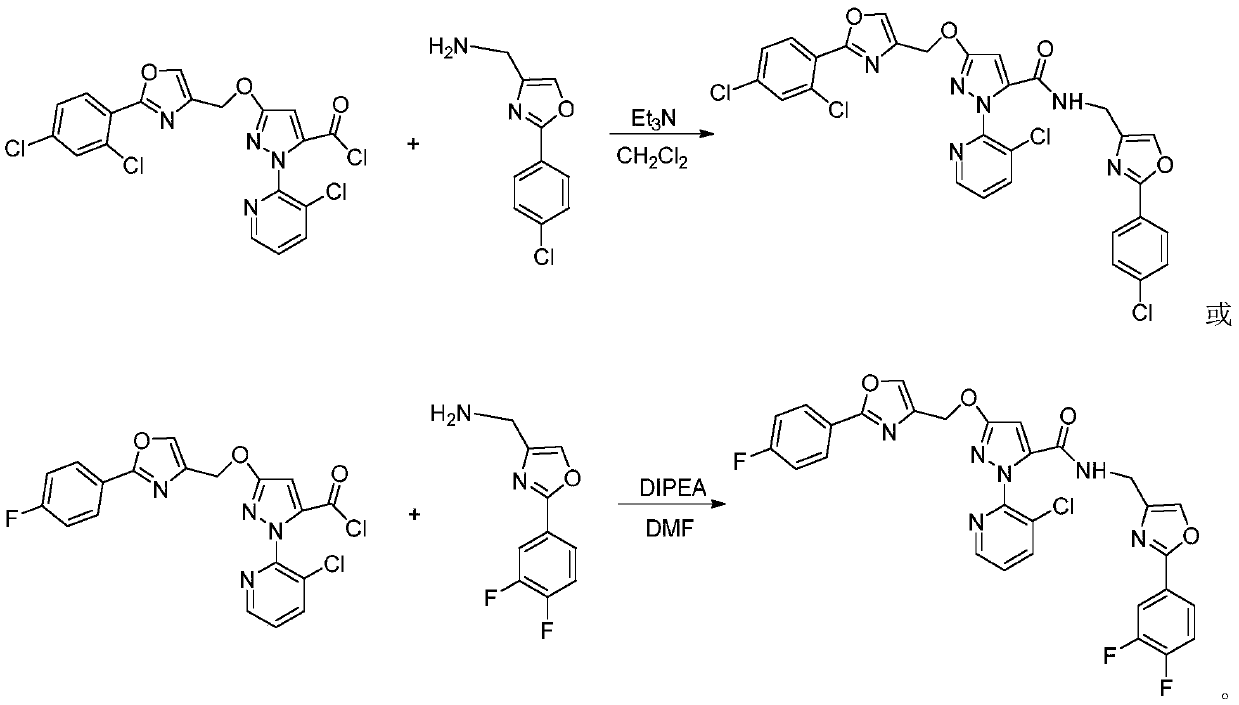
[0032] 3 mmol of compound IIIa was dissolved in 30 mL of dichloromethane, then 2 mL of triethylamine was added, and then 5 mmol of intermediate IIb was added thereto under ice-cooling conditions. After addition, the reaction was heated to reflux for 15 hours. The reaction was stopped, and the reaction solution was rotary evaporated to dryness under reduced pressure, and the resulting residue was separated and purified by column chromatography to obtain the target compound Ib; 1 H NMR (400MHz, CDCl 3 ):δ8.46~8.47(m,1H,Py-H),7.86~7.97(m,5H,Py-H,Ar-H and Oxazole-H),7.61(s,1H,Oxazole-H),7.51 (d, J=2.0Hz, 1H, Ar-H), 7.45(d, J=8.4Hz, 2H, Ar-H), 7.32-7.38(m, 2H, Py-H and Ar-H), 6.99( s,1H,NH),6.30(s,1H,Pyrazole-H),5.32(s,2H,CH 2 ), 4.45 (d, J=5.6Hz, 2H, CH 2 ).
Embodiment 3
[0034]
[0035] 6 mmol of compound IIIb was dissolved in 30 mL of DMF, then 15 mmol of DIPEA was added, and then 7 mmol of intermediate IIa was added thereto under ice-cooling conditions. After the addition was complete, the mixture was heated to 80°C for 20 hours. The reaction was stopped, and the reaction solution was rotary evaporated to dryness under reduced pressure, and the obtained residue was separated and purified by column chromatography to obtain the target compound Ic. 1 H NMR (400MHz, CDCl 3 ):δ8.46~8.48(m,1H,Py-H),8.01~8.05(m,2H,Ar-H),7.87~7.89(m,1H,Py-H),7.72~7.82(m,3H ,Ar-H and Oxazole-H),7.59(s,1H,Oxazole-H),7.35~7.38(m,1H,Py-H),7.21~7.26(m,1H,Ar-H),7.09~7.15 (m,3H,Ar-H and NH),6.29(s,1H,Pyrazole-H),5.28(s,2H,CH 2 ), 4.45 (d, J=5.6Hz, 2H, CH 2 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com