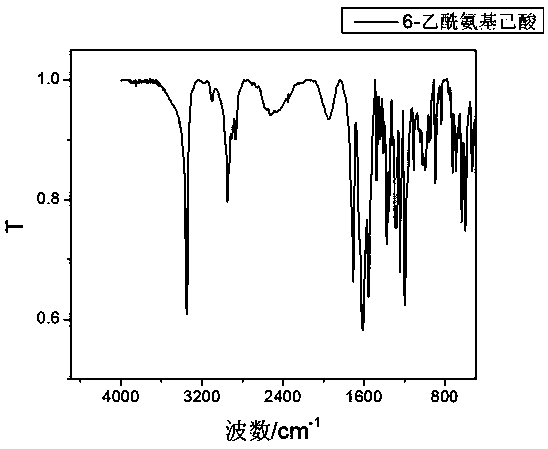
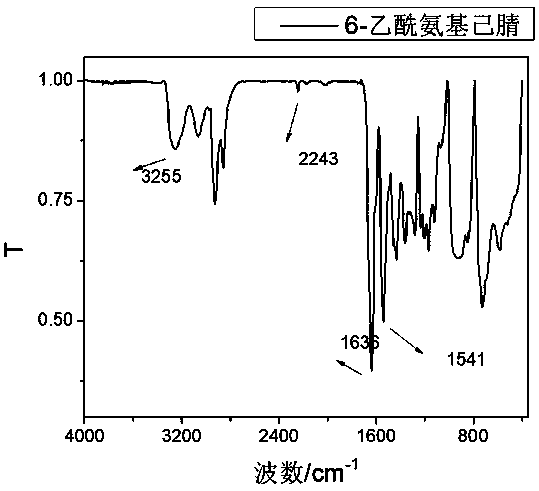
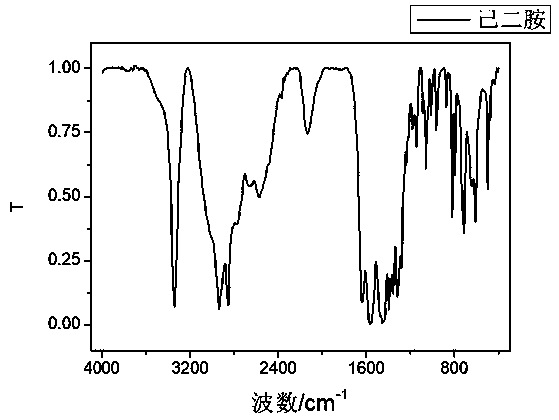
Method for synthesizing hexanediamine by taking caprolactam as raw material
A technology of caprolactam and hexamethylenediamine, which is applied to the preparation of carboxylic acid amide, chemical instruments and methods, and the preparation of organic compounds, can solve the problems of undisclosed yield of hexamethylenediamine, low yield, difficult control, etc., and achieve The effect of easy equipment requirements, convenient post-processing, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] A kind of method taking caprolactam as raw material synthetic hexamethylenediamine comprises the following steps:
[0032] 1) Add 10g caprolactam into a 250ml single-mouth bottle, add 11g sodium hydroxide and 25ml water, reflux at 130°C for 3h and cool to room temperature to obtain 6-aminocaproic acid sodium salt; the molar ratio of caprolactam and sodium hydroxide is about is 1:3; the molar ratio of caprolactam to the amount of water added is 1:15.8;
[0033] 2) Then add 9ml of acetic anhydride (amino-protecting group, the amount of amino-protecting group is 1 times the equivalent of caprolactam) to the one-necked bottle of step 1), then after reaching 47°C, stir the reaction for 2 hours, then add concentrated hydrochloric acid to adjust The pH value of the solution is at 2, and the precipitated solid is recrystallized with water to obtain 6-acetylaminocaproic acid;
[0034] 3) Dry the product of step 2) to obtain 12g of solid; take 5g of the dried product and 0.15g o...
Embodiment 2
[0037] A kind of method taking caprolactam as raw material synthetic hexamethylenediamine comprises the following steps:
[0038] 1) Add 10g caprolactam into a 250ml single-mouth bottle, add 13g sodium tert-butoxide and 32ml water, reflux at 150°C for 2h and cool to room temperature to obtain 6-aminocaproic acid sodium salt; The ratio is about 1:1.5; the molar ratio of caprolactam to the amount of water added is 1:20;
[0039]2) Then slowly add 18ml of benzyl chloroformate (amino-protecting group, the amount of which is 1.5 times the equivalent of caprolactam) to the single-necked bottle of step 1), stir and react for 2 hours at room temperature, and then add to the reaction solution Extract the by-products in the reaction with 30ml of ether, repeat the operation twice, then add concentrated hydrochloric acid to the extracted aqueous layer solution under ice bath to adjust the pH to 5; put it in the refrigerator (minus 3°C) for 2h and filter to obtain a white solid N -Cbz-ami...
Embodiment 3
[0043] A kind of method taking caprolactam as raw material synthetic hexamethylenediamine comprises the following steps:
[0044] 1) Add 10g caprolactam into a 250ml single-necked bottle, add 7g sodium hydroxide and 16ml water, reflux at 100°C for 4h and cool to room temperature to obtain 6-aminocaproic acid sodium salt; the molar ratio of caprolactam and sodium hydroxide is about is 1:2; the molar ratio of caprolactam to the amount of water added is 1:10;
[0045] 2) Then slowly add 15ml of tetrahydrofuran and 20ml of benzyl chloride (amino-protecting group, the amount of amino-protecting group is twice the equivalent of caprolactam) to the single-necked bottle of step 1), reflux at 70°C for 10h, and cool to room temperature , adding concentrated hydrochloric acid to the obtained solution to adjust the pH value of the solution to 3; adding 20ml of ethyl acetate after the reaction to extract the product, and repeating the operation 3 times. The obtained ethyl acetate layer wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com