Compositions for reducing sarcolipin expression and preventing and treating muscular dystrophy and cardiomyopathy and methods of use
A composition, recombinant virus technology, applied in the fields of molecular biology, virology, and medicine, can solve problems such as incurable DMD
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0087] The compositions, vectors, viruses and methods described herein can be used to reduce SLN expression in any tissue in which SLN is elevated. For example, because SLN is also elevated in the ventricles of patients with mitral regurgitation and in the muscles of patients with Tako-Tsubo cardiomyopathy and in patients with Emery-Dreifuss muscular dystrophy, the SLN could serve as a target for these diseases, and SLN expression can be reduced by administering the compositions, viruses and vectors described herein.
[0088] effective dose
[0089] The compositions, viruses, and vectors described herein are preferably present in effective amounts (i.e., capable of producing desired results (e.g., reducing SLN expression, restoring SERCA function, improving LV systolic function and cardiac remodeling, improving forelimb muscle) in the treated mammal. Strength, life-prolonging amount)) administered to mammals (eg, humans). Such a therapeutically effective amount can be determ...
Embodiment 1
[0097] Example 1 - Reduction of Sarcolipin Expression Alleviates DMD and Associated Cardiomyopathy in Mice
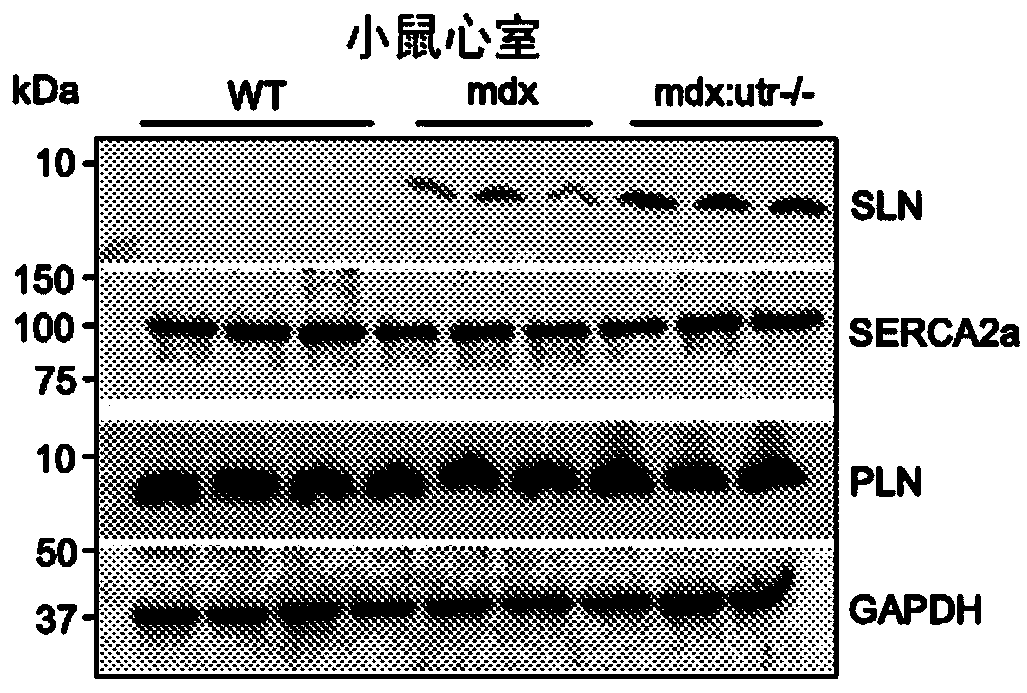
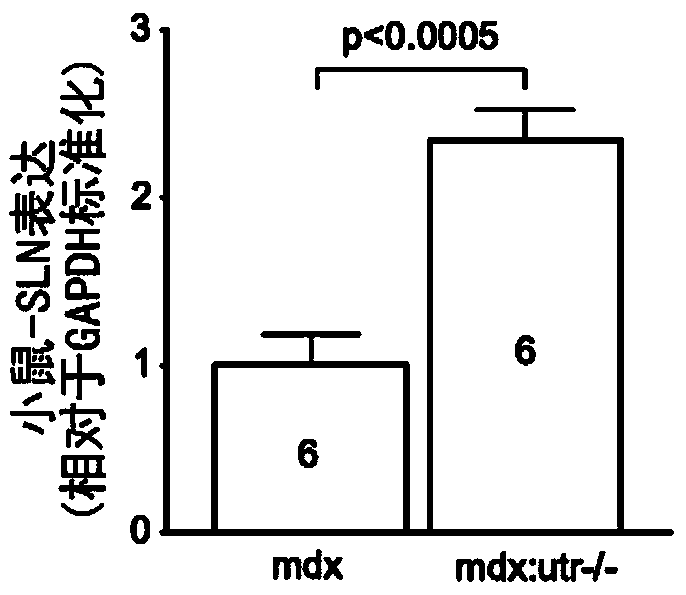
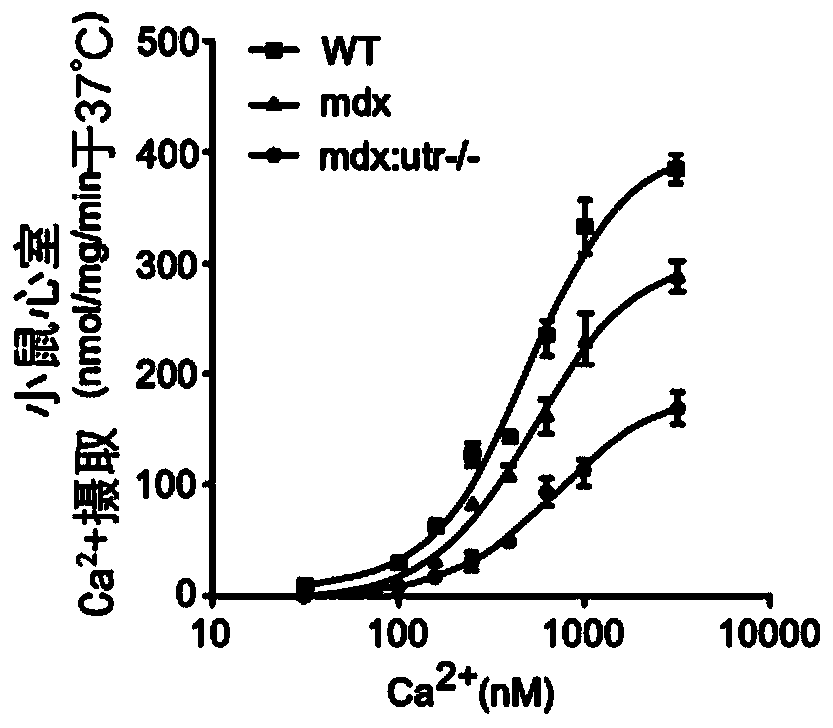
[0098] Here it is shown that reduction of SLN levels ameliorates dystrophic pathology in a severe dystrophin / utrophin double mutant (mdx:utr- / -) mouse model of DMD. Germline inactivation of one-allele of the SLN gene normalized SLN expression, restored SERCA function, attenuated skeletal muscle and cardiac pathology, improved muscle regeneration, and extended lifespan. To translate these findings into therapeutic strategies, SLN expression was knocked down by AAV9-mediated RNA interference in one-month-old mdx:utr- / - mice. AAV treatment significantly reduced SLN expression, attenuated muscle pathology and improved diaphragmatic, skeletal muscle and cardiac function. Taken together, these findings suggest SLN reduction as a therapeutic approach for DMD.
[0099] To determine whether SLN upregulation is a common molecular change in skeletal muscle and heart in DMD, SLN ...
Embodiment 2
[0134] Example 2 - Increased SLN protein levels in canine dystrophic myoblasts
[0135] SLN was upregulated in dystrophic canine myoblasts ( Figure 16 ). Canine-specific shSLN expressed using AAV9 (5'-ctgtttctcaacttcactattgtttcaagagaacaatagtgaagttgagaaacag-3') (SEQ ID NO:5) reduced SLN expression in dystrophic canine myoblasts ( Figure 17 ).
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap