Inhibitor of heat shock factor 1, and preparation method and application of inhibitor
A -C1-C10, unsubstituted technology, applied in the field of medicinal chemistry and pharmacotherapeutics, can solve the problem that there are not many HSF1 inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0159] Example 1: Dorsomorphin inhibits the expression of heat shock proteins induced by various exogenous stimuli and endogenous in tumor cells
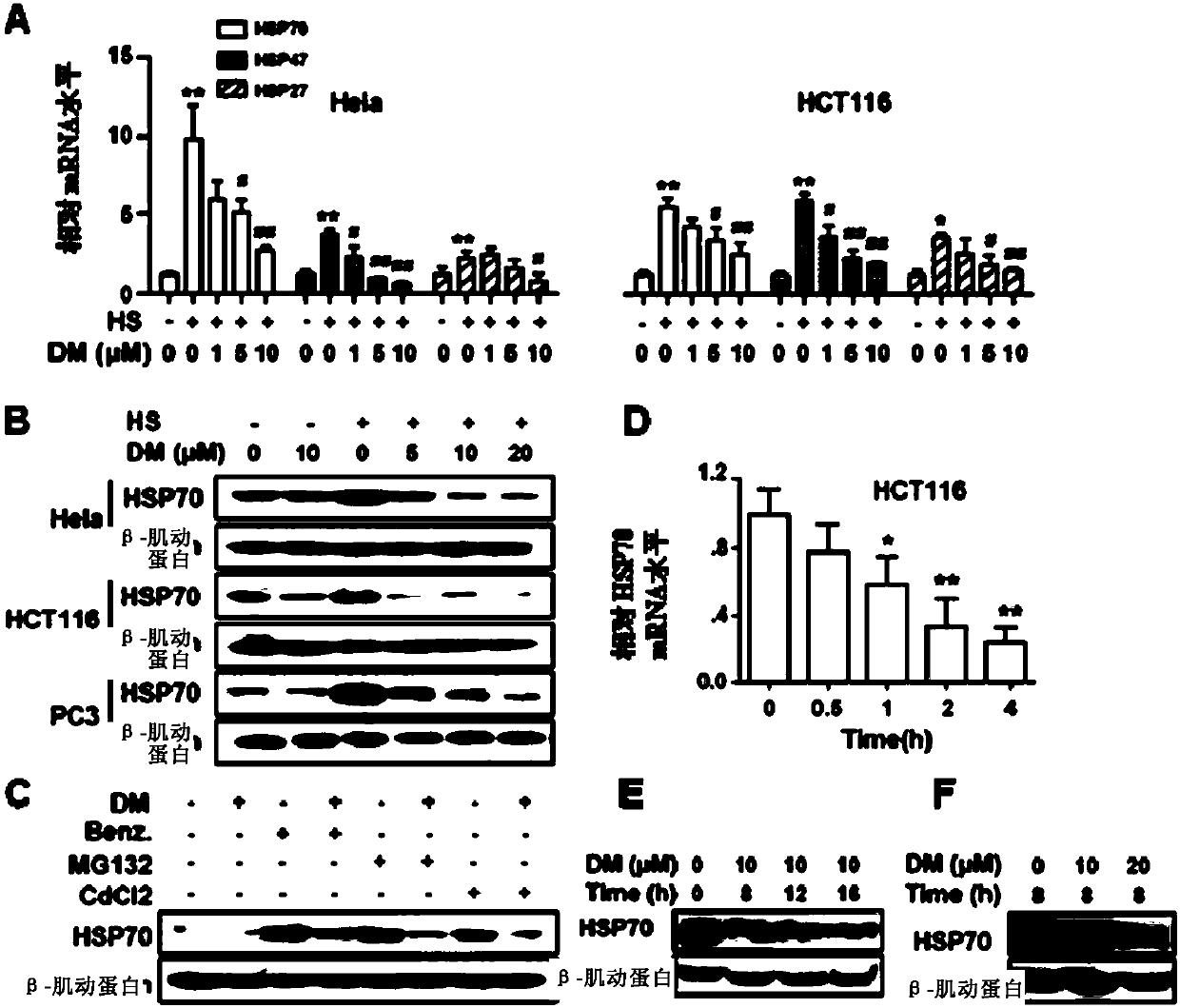
[0160] First, by detecting the effect of Dorsomorphin on the expression of heat shock proteins induced by heat stimulation in cervical cancer cell line Hela and colon cancer cell line HCT116, it can be found that Dorsomorphin can concentration-dependently inhibit the induction of heat shock proteins (including HSP70, HSP47 and HSP27) at the mRNA level ( figure 1 A), and confirmed that Dorsomorphin can concentration-dependently inhibit heat stimulation-induced expression of HSP70 at the protein level in Hela, HCT116 and prostate cancer cell line PC3 ( figure 1 B).
[0161] Secondly, taking HSP70 as the representative of heat shock proteins, the inventors detected the effect of Dorsomorphin on the expression of heat shock proteins induced by other stimuli, and found that Dorsomorphin can also inhibit HSP90 inhibitors Benzisoxazoles (...
Embodiment 2
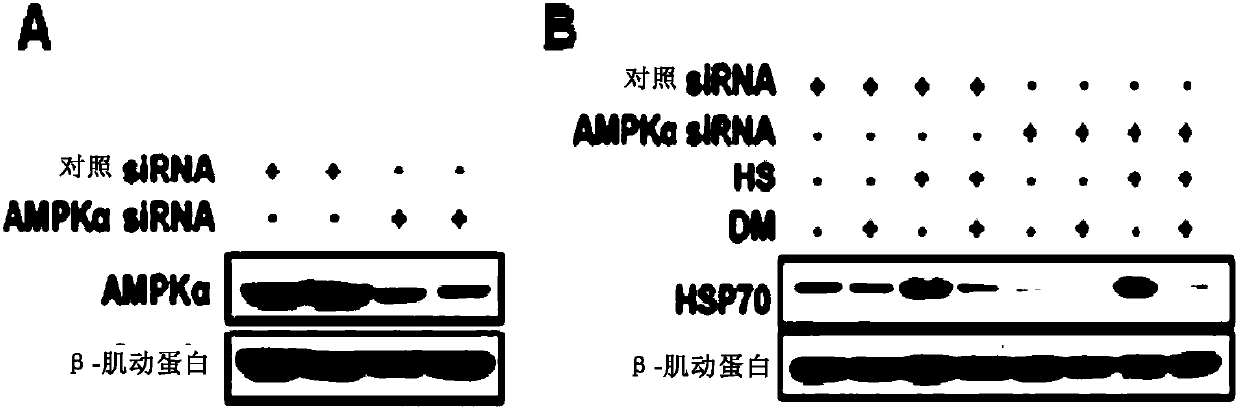
[0164] Example 2: The inhibitory effect of Dorsomorphin on the expression of HSP70 induced by heat stimulation is not dependent on AMPK.
[0165] Dorsomorphin is a commonly used AMPK inhibitor. The inventor knocked down AMPKα ( figure 2 A), it was found that AMPK knockdown had no effect on Dorsomorphin's inhibition of HSP70 expression induced by heat stimulation ( figure 2 B), indicating that AMPK is not involved in the inhibition of HSP70 protein expression by Dorsomorphin.
Embodiment 3
[0166] Example 3: Dorsomorphin inhibits HSF1 nuclear translocation
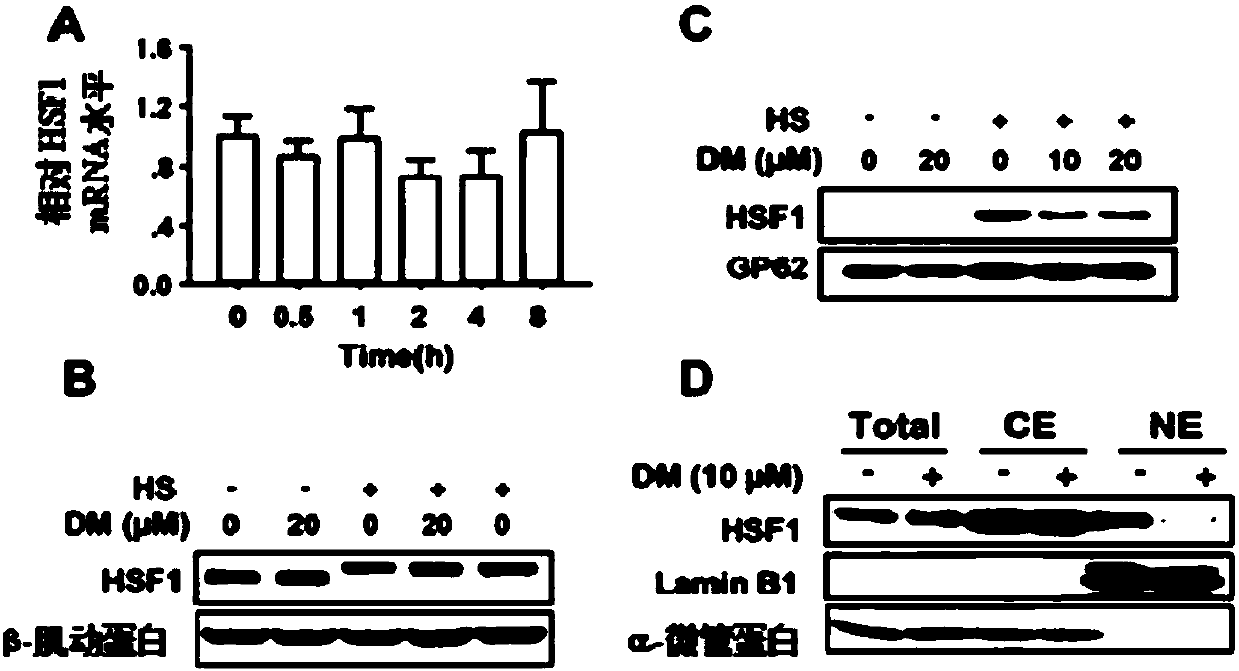
[0167] The expression of HSPs is mainly regulated by the transcription factor HSF1, and the regulation of HSF1 activity mainly includes the following aspects, first, the expression of HSF1, including the mRNA level and protein level; second, the phosphorylation of HSF1, and the activity of HSF1 is controlled by various transcription factors post-modification regulation, especially its phosphorylation. Third, HSF1 needs to form a trimer to enter the nucleus, and bind to the DNA promoter HSE region to initiate the transcription of downstream target genes.
[0168] The experimental results showed that Dorsomorphin did not affect the expression of tumor cell HSF1 at the mRNA and protein levels ( image 3 A-B), also did not affect heat-induced HSF1 phosphorylation ( image 3 B), but it can significantly inhibit the nuclear translocation of HSF1 induced by heat stimulation ( image 3 C). In tumor cells, Dorsomo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com