A kind of production technology of recombinant trypsin
A production process, trypsin technology, applied in the field of recombinant trypsin purification production process, can solve the problems of low yield of recombinant trypsin, low renaturation rate of inclusion body, complicated purification process, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
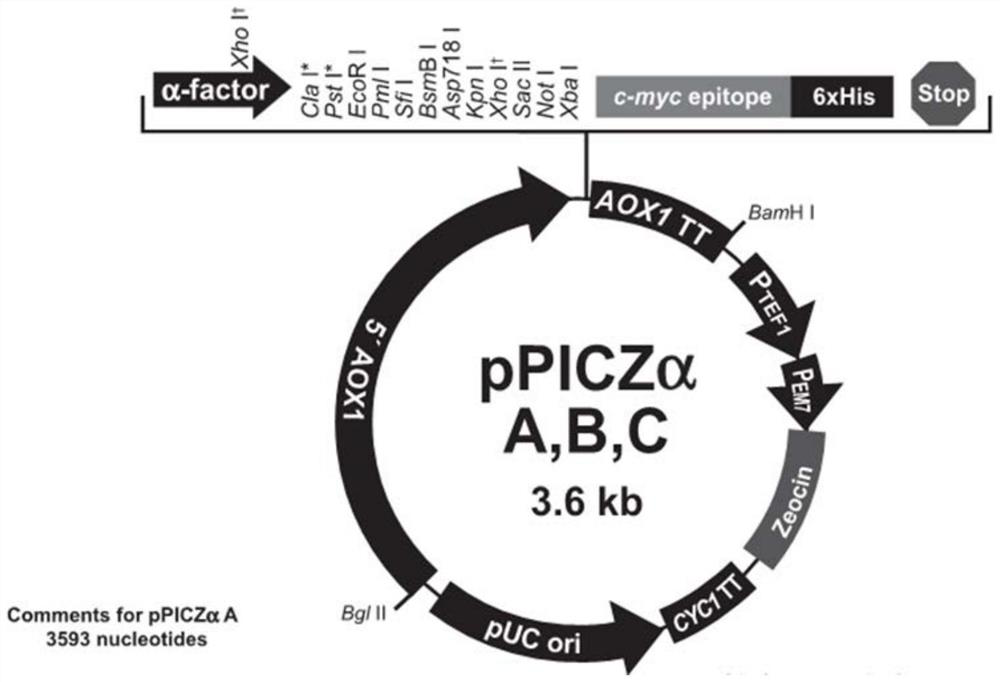
[0036] First, it is translated into a gene sequence (its sequence is shown in Seq ID No: 3) according to the porcine trypsinogen sequence (its sequence is shown in Seq ID No: 1), and the nucleic acid corresponding to the EK restriction site is contained at its 5' end sequence and 5'XhoI (ctcgag) restriction site, and a terminator and 3'NotI (gcggccgc) restriction site are added at the 3' end. The above sequence was synthesized by a gene synthesis service company, and cloned into the pGEM-T (promega company) vector. After sequencing verification, the sequence of the gene was complete and correct. Then extract the plasmids separately, use XhoI / NotI double enzymes to digest PGEMT to recover the porcine trypsin gene; use XhoI / NotⅠ double enzymes to digest pPICZαA yeast expression gene to recover a large fragment, and then connect the above two fragments to form a gene containing the yeast α-factor The recombinant yeast expression plasmid pPICZαA in the form of leader peptide-enter...
Embodiment 2
[0037] The expression fermentation of embodiment 2 porcine trypsin
[0038] Use the inoculation loop to pick the porcine trypsin engineering expression strain, put it into YPG medium (6 grams of tryptone, 3 grams of yeast powder, 6 grams of glycerol in a 5-liter glass beaker, set the volume to 300 milliliters with purified water, Packed in a 1000mL Erlenmeyer flask). Put the inoculated YPG shaker flask into a shaker, and cultivate it at 220rpm for about 18-24 hours, so that OD600≥5, as the seed solution.
[0039] Pour the seed liquid into the fermenter, supplement the yeast medium, and adjust the ventilation flow to not less than 10L / min. The condition control of the fermentation culture stage is as follows: the temperature is controlled at 20-30° C.; the pH is controlled at 4.0-5.0. Check and record every 0.5-2 hours. The records include: temperature, pH, dissolved oxygen PO2, stirring Stirr, ventilation Air, alkali volume, ventilation volume, and feed volume. When cultiva...
Embodiment 3
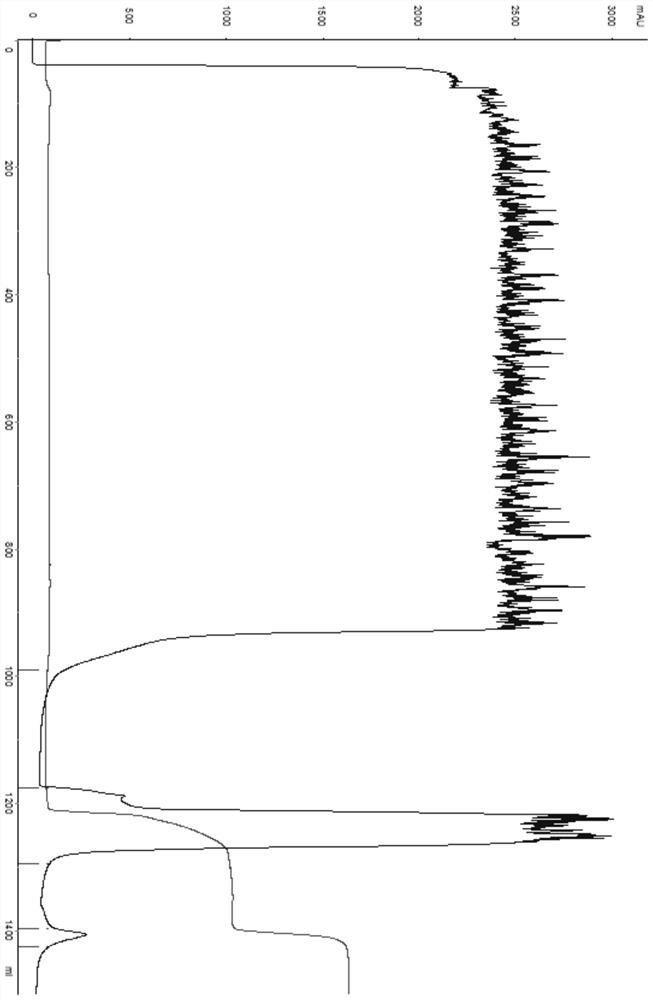
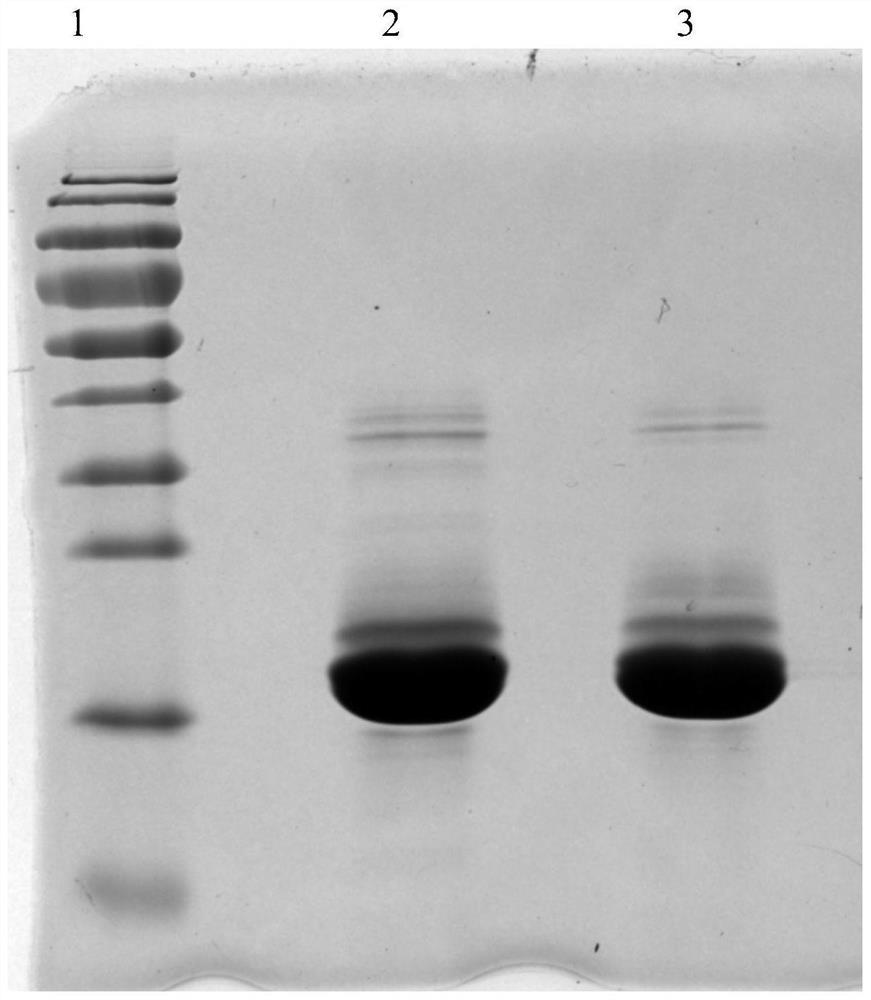
[0041] The purification of embodiment 3 porcine trypsin
[0042] 1) Sample pretreatment: add 1 times of purification water to the fermentation supernatant, and the conductance of the final sample solution is about 7.0ms / cm; the pH is controlled at about 4.5.
[0043] 2) Chromatographic column equilibrium: a chromatographic column with a diameter of 25 cm, and the filler is an ion exchange column SP Bestarose FF (Borgeron (Shanghai) Biotechnology Co., Ltd.). Use equilibration buffer (50mM NaAc-HAc, 2mM CaCl2, pH4.5;) to equilibrate 3CV, conductance and pH baseline go flat.
[0044] 3) Sample loading: take the pretreated fermentation broth and load the sample.
[0045] 4) Chromatographic column re-equilibration: equilibrate 2CV with equilibration buffer.
[0046] 5) Sample elution: elute the target peak with eluent (50mM NaAc-HAc, 0.4M NaCl, 2mM CaCl2, pH4.5).
[0047] 6) Chromatographic column regeneration: first wash 1CV with 1mol / L NaOH, then wash 1CV with eluent, and then...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap