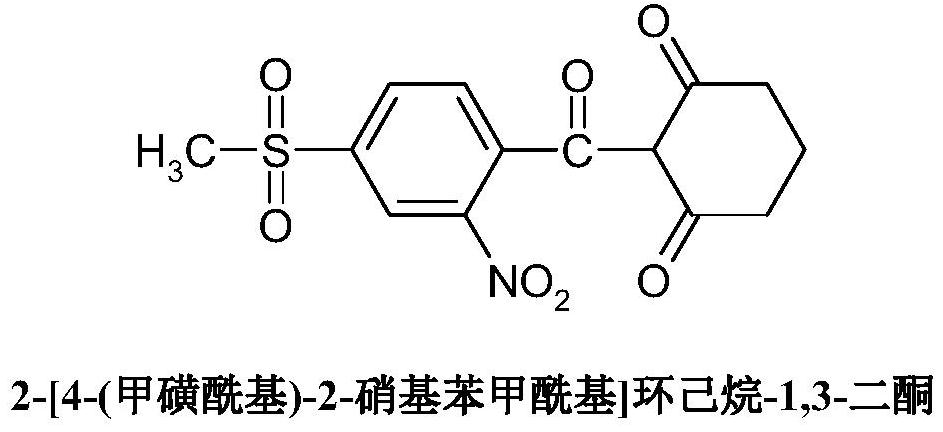
Synthetic method of methylsulfonone ketone
A technology of mesotrione and mesotrione, which is applied in organic chemistry methods, chemical instruments and methods, preparation of organic compounds, etc., can solve problems such as corrosiveness and unfriendly environment.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
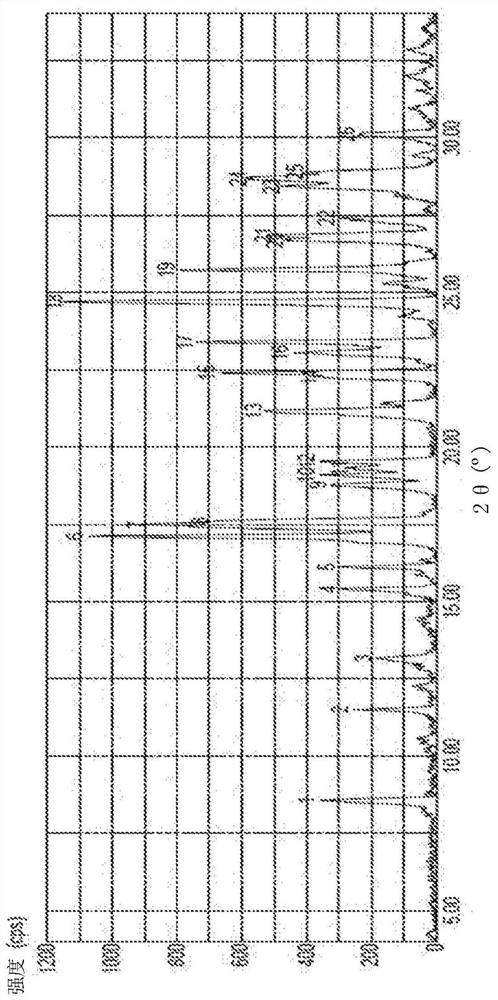
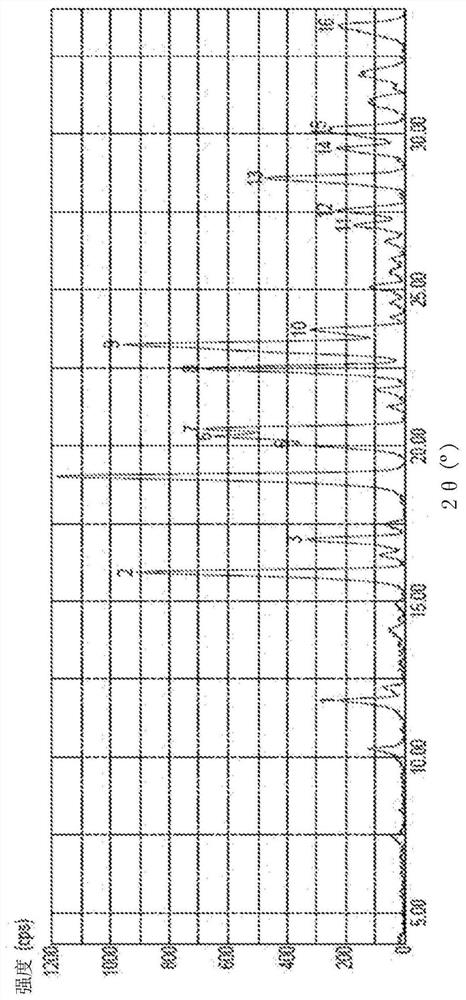
Image
Examples
preparation example Construction
[0038] According to the present disclosure, the synthesis method of crystalline mesotrione comprises the following steps:
[0039] i. reacting p-toluenesulfinyl chloride with at least one alkali metal sulfite and at least one alkali metal bicarbonate to obtain an alkali metal p-toluenesulfinate, which is combined with reacting at least one alkali metal monochloroacetic acid salt to obtain p-methylsulfonyltoluene;
[0040] ii. nitrating p-methylsulfonyltoluene using a mixture of sulfuric acid and at least one nitrating agent to obtain 2-nitro-p-methylsulfonyltoluene;
[0041] iii. Oxidizing 2-nitro-p-methylsulfonyltoluene using at least one oxidizing agent in the presence of at least one first catalyst to obtain 2-nitro-p-methylsulfonylbenzoic acid;
[0042] iv. Halogenating 2-nitro-p-methylsulfonylbenzoic acid using at least one halogenating agent in a first fluid medium to obtain 2-nitro-p-methylsulfonylbenzoyl halide;
[0043] v. In the presence of a second fluid medium an...
Embodiment approach
[0101] Typically, the base is selected from the group consisting of triethylamine, sodium hydride and 1,2,4-triazole. According to an exemplary embodiment of the present disclosure, the base is triethylamine, and the third fluid medium is 1,2-dichloroethane.
[0102] The material comprising mesotrione is processed using known techniques to obtain mesotrione with a purity of less than or equal to 99%.
[0103] Step Seven: Converting Amorphous Mesotrione to Crystalline Mesotrione
[0104] method 1
[0105] In the first step, the amorphous mesotrione is dissolved using a fourth fluid medium to obtain a solution. In an exemplary embodiment of the present disclosure, the fourth fluid medium is 1,2-dichloroethane.
[0106] In a second step, the solution is treated with charcoal to obtain a first mixture.
[0107] In the third step, a part of the fourth fluid medium is distilled off from the first mixture to obtain a second mixture comprising the remaining part of the fourth ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap