Targeted nano-preparation modified by mannose
A nano-formulation, mannose technology, applied in the field of medicine, can solve the problems of large particle size of liposomes, difficult to control conditions, and inability to guarantee the exposure of mannose, and achieve precise targeted therapy and good tumor targeting. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
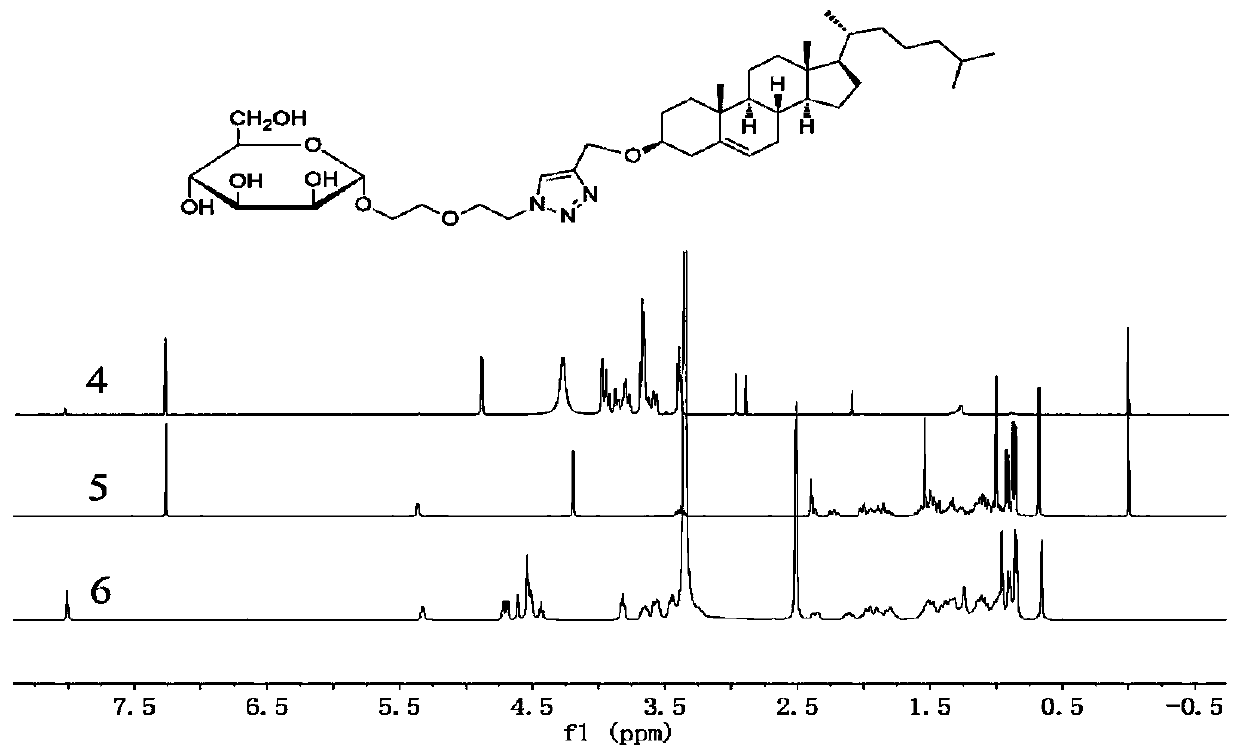
[0083] The synthesis of embodiment 1 carrier material
[0084] Mannose-PEG n -Chol: Taking the synthesis of mannose-PEG100-Chol as an example, other PEGs of different lengths are also synthesized according to this synthesis route. Take diethylene glycol, p-toluenesulfonyl chloride and triethylamine, dissolve in DCM, react at room temperature for 24 hours, and separate the product TosOC by silica gel column chromatography 2 h 4 OC 2 h 4 OH (Formula I). Take the compound of formula I with pentaacetylated mannose and boron trifluoride ether (BF 3 ·Et 2 O), be dissolved in DCM, room temperature reaction 24h, silica gel column chromatography separates and obtains product Aco-Mannose-OC 2 h 4 OC 2 h 4 OTos (Formula II). Take the compound of formula II and sodium azide, dissolve in DMF, react at 60°C for 24 hours, and separate by silica gel column chromatography to obtain the solid product Aco-Mannose-OC 2 h 4 OC 2 h 4 N 3 (Formula III). Dissolve the compound of form...
Embodiment 2
[0089] The preparation of embodiment 2 liposomes
[0090] prescription composition
[0091]
[0092]
[0093] Note: The main drug of prescription 1 to prescription 5 is GFP-mRNA.
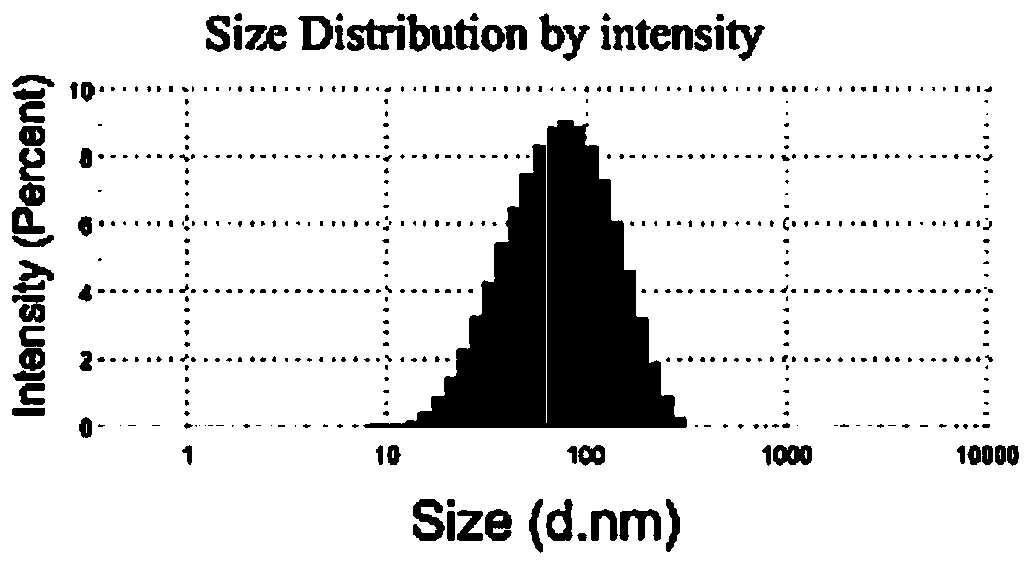
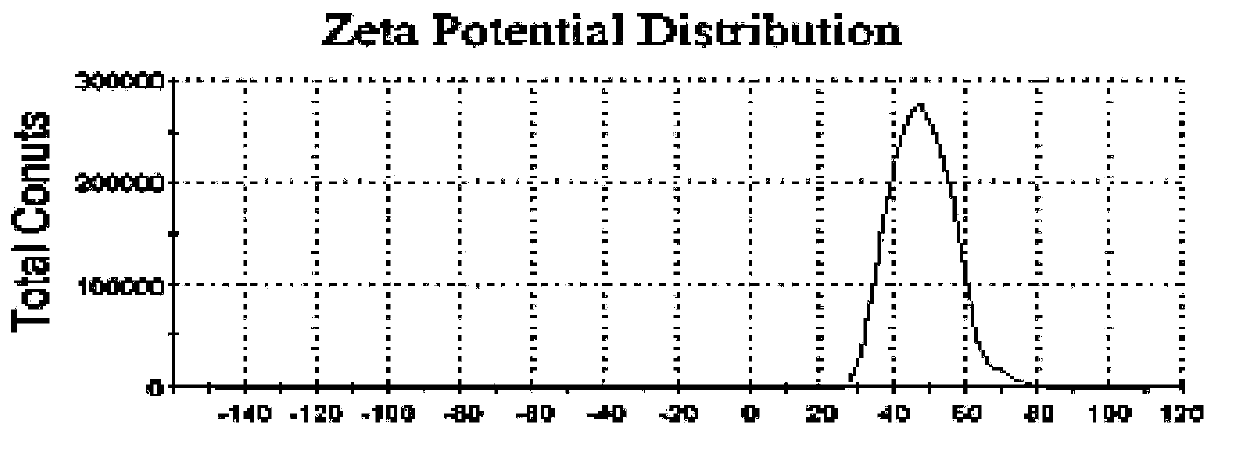
[0094] Preparation method 1: Take phospholipids in different weight ratios according to the above prescription (when the main drug is PTX, add them together with phospholipids), add them to a round bottom flask, add chloroform / ethanol=1:1 (v / v) to dissolve, The organic solvent was removed by rotary evaporation under reduced pressure, and a thin film was formed on the inner wall of the bottle. PBS7.4 buffer solution was added for hydration, and 100W ultrasonic wave was used for 3 minutes to obtain liposomes. When encapsulating pGFP, it can be obtained by incubating blank liposomes with pGFP at room temperature, and the nanoparticles obtained from mannose-PEG1000-Chol can be recorded as MP1000-LPX. The liposome obtained is characterized, and the result shows that the particle size of liposome ...
Embodiment 3
[0097] The preparation of embodiment 3 solid fat nanoparticles
[0098] Solid lipid nanoparticles are nanostructured carriers prepared from lipids as the skeleton material, and have the characteristics of physiological compatibility, cell affinity, and targeting. In this example, adding mannose-modified lipids improves the targeting of solid lipid nanoparticles.
[0099] prescription composition
[0100]
[0101]
[0102] Preparation method: Precisely weigh the prescribed amount of medicines and lipid materials, dissolve them in ethanol, stir in a constant temperature water bath to form an oil phase; weigh the prescribed amount of surfactants, dissolve them in pure water, and heat them in a constant temperature water bath to form a water phase; , inject the oil phase into the water phase, stir and emulsify and concentrate at a constant temperature. After the emulsion is concentrated to a certain volume, pour it into cold water at a constant temperature of 4°C, stir and ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap