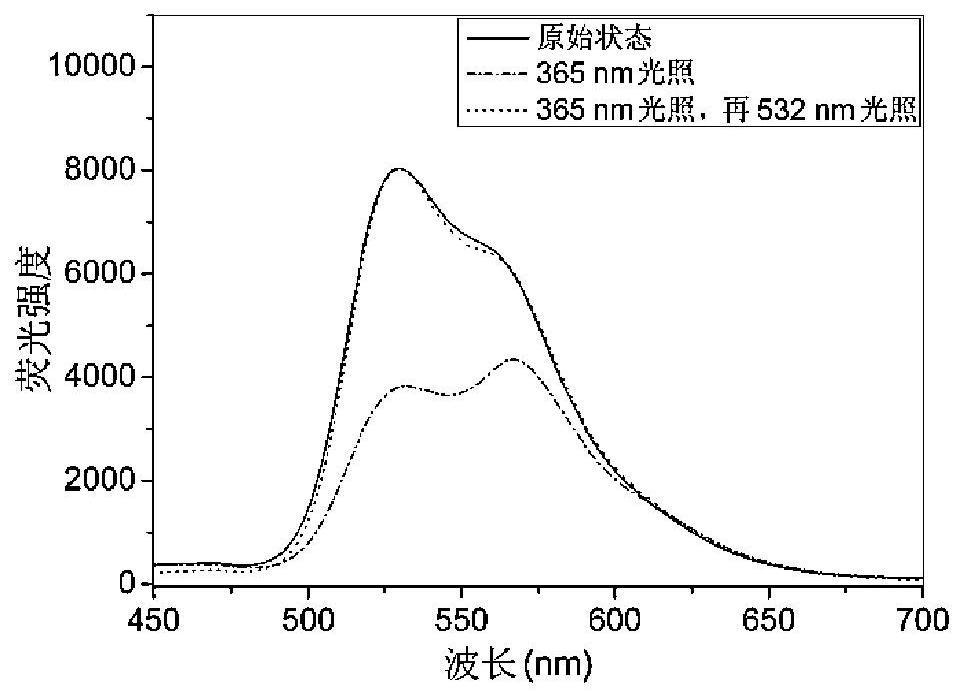
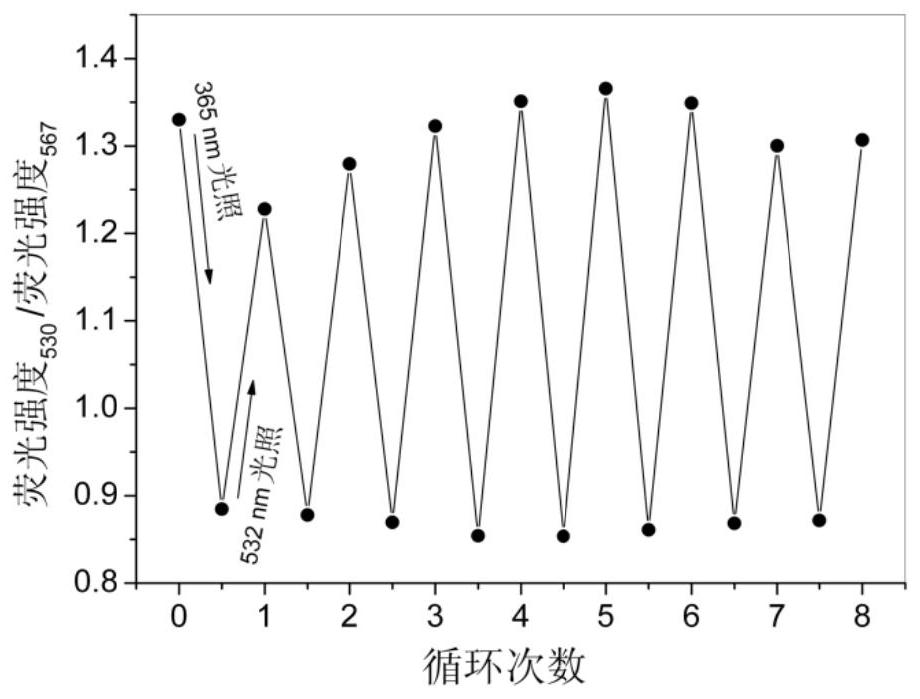
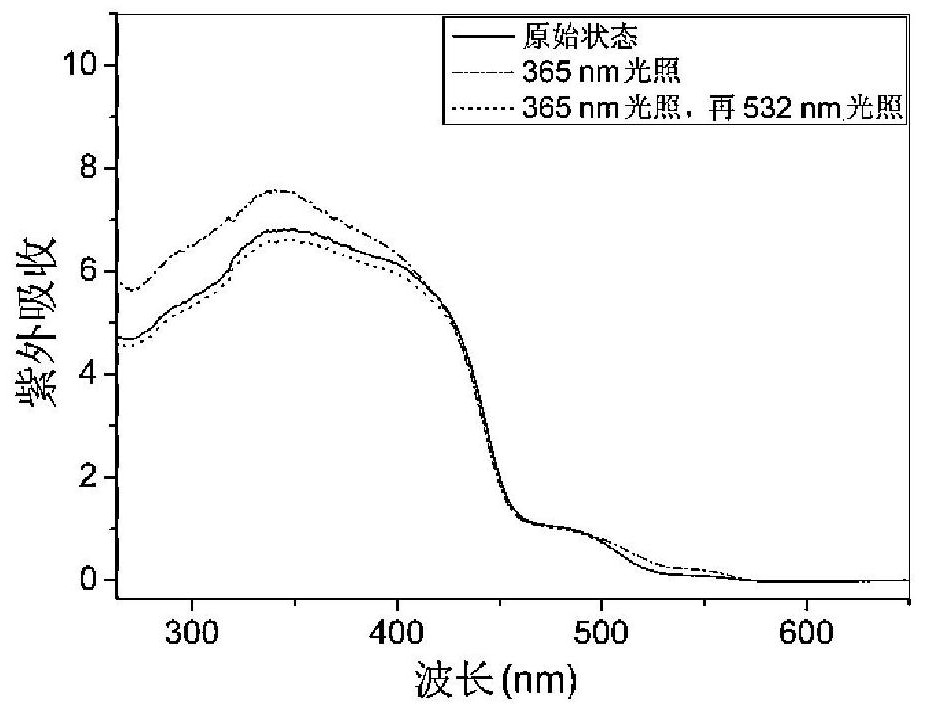
Bile acid-salicylaldehyde aniline conjugate, preparation method and application thereof, and solid-state photoluminescent color-changing anti-counterfeiting method based on it
A technology of salicylaldehyde aniline and conjugates, which is applied in the fields of cholic acid-salicylaldehyde aniline conjugates, solid-state photofluorescence color change anti-counterfeiting, and can solve the problems that the molecular conformation has a great influence and limits practical applications.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0111] The preparation method of cholic acid-salicylaldehyde aniline conjugate of the present invention comprises the following steps:
[0112] 1) Weigh cholic acid, 2,4-dihydroxybenzaldehyde, 4-dimethylaminopyridine, dicyclohexylcarbodiimide and dichloromethane, and mix cholic acid, 2,4-dihydroxybenzaldehyde, 4 -Dimethylaminopyridine and dicyclohexylcarbodiimide are dispersed in dichloromethane to obtain reaction mixture a, in which, cholic acid, 2,4-dihydroxybenzaldehyde, 4-dimethylaminopyridine, dicyclohexylcarbodiimide The ratio of diimine and dichloromethane is 2.0g: 1.0g: 61mg: 1.5g: 10mL;
[0113] 2) The reaction mixture a obtained in step 1) was stirred and reacted at room temperature. After the reaction was completed, it was filtered and the solvent was removed, and the cholic acid-salicylaldehyde conjugate was obtained as a white powder solid after elution by column chromatography;
[0114] 3) Weigh the cholic acid-salicylaldehyde conjugate, 4,4'-diaminobiphenyl and...
Embodiment 2
[0123] The preparation method of cholic acid-salicylaldehyde aniline conjugate of the present invention comprises the following steps:
[0124] 1) Weigh cholic acid, 2,4-dihydroxybenzaldehyde, 4-dimethylaminopyridine, dicyclohexylcarbodiimide and dichloromethane, and mix cholic acid, 2,4-dihydroxybenzaldehyde, 4 -Dimethylaminopyridine and dicyclohexylcarbodiimide are dispersed in dichloromethane to obtain reaction mixture a, wherein, cholic acid, 2,4-dihydroxybenzaldehyde, 4-dimethylaminopyridine, dicyclohexylcarbodiimide The ratio of diimine and dichloromethane is 2.0g:0.7g:183mg:1.0g:50mL;
[0125] 2) The reaction mixture a obtained in step 1) was stirred and reacted at room temperature. After the reaction was completed, it was filtered and the solvent was removed, and the cholic acid-salicylaldehyde conjugate was obtained as a white powder solid after elution by column chromatography;
[0126] 3) Weigh the cholic acid-salicylaldehyde conjugate, 4,4'-diaminobiphenyl and eth...
Embodiment 3
[0135] The preparation method of cholic acid-salicylaldehyde aniline conjugate of the present invention comprises the following steps:
[0136] 1) Weigh cholic acid, 2,4-dihydroxybenzaldehyde, 4-dimethylaminopyridine, dicyclohexylcarbodiimide and dichloromethane, and mix cholic acid, 2,4-dihydroxybenzaldehyde, 4 -Dimethylaminopyridine and dicyclohexylcarbodiimide are dispersed in dichloromethane to obtain reaction mixture a, in which, cholic acid, 2,4-dihydroxybenzaldehyde, 4-dimethylaminopyridine, dicyclohexylcarbodiimide The ratio of diimine and dichloromethane is 2.0g: 0.85g: 100mg: 1.3g: 30mL;
[0137] 2) The reaction mixture a obtained in step 1) was stirred and reacted at room temperature. After the reaction was completed, it was filtered and the solvent was removed, and the cholic acid-salicylaldehyde conjugate was obtained as a white powder solid after elution by column chromatography;
[0138] 3) Weigh the cholic acid-salicylaldehyde conjugate, 4,4'-diaminobiphenyl a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com