Flavonoid derivatives and preparation method thereof and application in preparation of immunoenhancer
A technology of immune enhancers and derivatives, which is applied in the fields of drug combination, organic chemistry, allergic diseases, etc., and can solve problems such as undiscovered immune enhancers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1: The synthetic route of flavonoid derivative YSW-01 is as follows:
[0034]
[0035] Add flavonoid derivative b (678mg, 1mmol), o-benzyloxybenzoic acid a (273.6mg, 1.2mmol), DMAP (183mg, 1.5mmol) into a 100ml one-port bottle, add 25ml of DMF, the system is in a suspended state, and finally add EDCI (287mg, 1.5mmol), after 1-2 hours, the system slowly dissolves, stir at room temperature overnight for 14h, TLC detects the reaction, after the basic reaction of the raw materials is complete, the system is poured into water, a yellow solid is precipitated, suction filtration, column chromatography For purification, petroleum ether was added, ultrasonication, beating at room temperature, and filtration to obtain 600 mg of a yellow solid with a yield of 67.5%.
[0036] 1 H NMR (500MHz, dmso-d 6 )δ12.11(s, 1H), 8.19(d, J=7.8Hz, 1H), 7.69(d, J=8.6Hz, 1H), 7.51(d, J=7.5Hz, 2H), 7.46-7.20( m, 26H), 7.16(t, J=7.6Hz, 1H), 6.98(s, 1H), 6.61(d, J=1.7Hz, 1H), 5.30(s,...
Embodiment 2
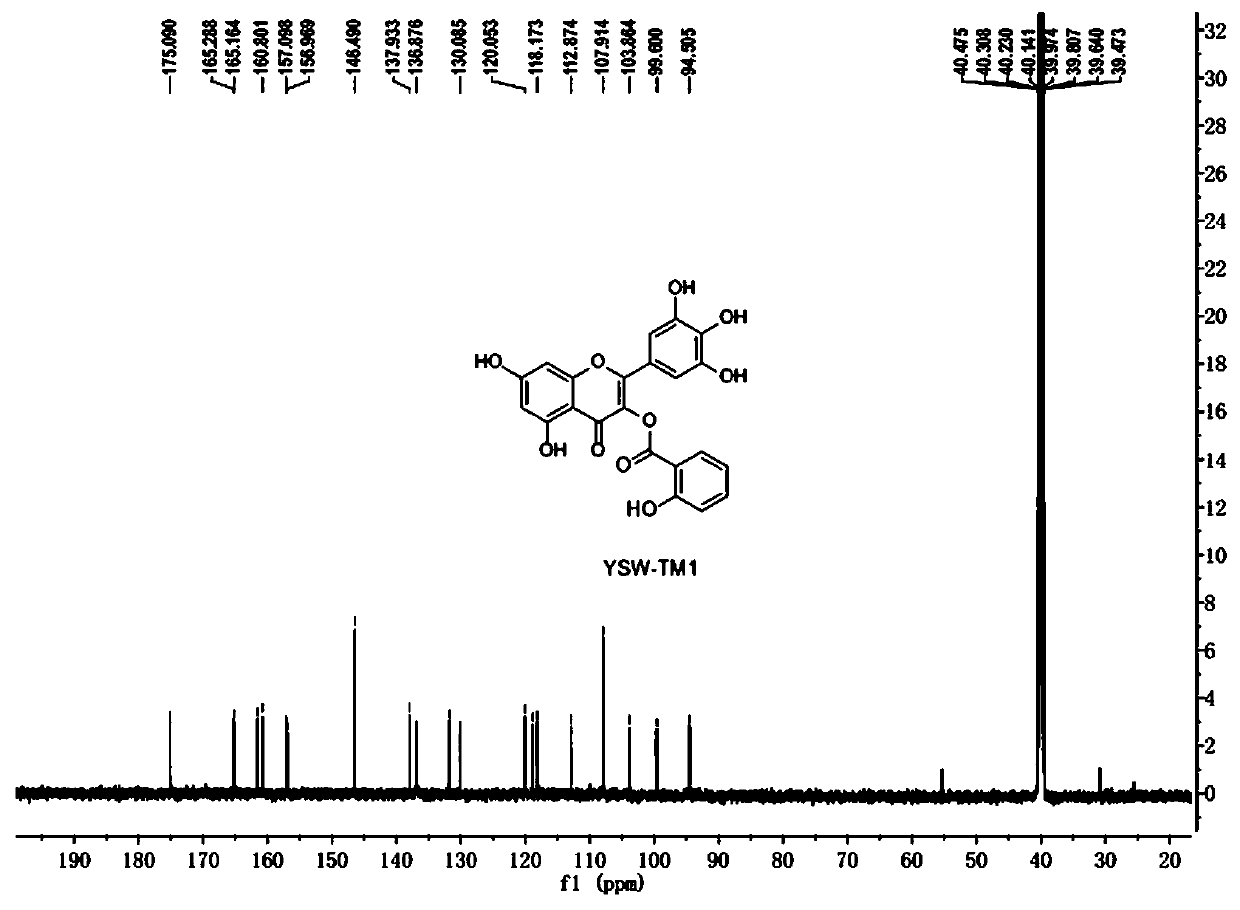
[0037] Example 2: The synthetic route of flavonoid derivative YSW-TM1 is as follows:
[0038]
[0039] The YSW-01 (600 mg, 0.675 mmol) prepared in Example 1 was dissolved in about 20 ml of MeOH, 100 mg of 10% Pd / C was added, and the double-layered hydrogen balloon was reacted overnight at room temperature, TLC (PE:EA=3: 1) The disappearance of raw materials was detected, and the main product was detected by LCMS. A small amount of diatomaceous earth was filtered to remove Pd-C to obtain a red solution, and the solvent was evaporated to dryness to obtain a yellow oil, which was purified by column chromatography to obtain 200 mg of a yellow solid with a yield of 67.8%. LCMS Detection ESI(-)[M-H]=437.
[0040] 1 H NMR (500MHz, dmso-d 6 )δ12.14(s, 1H), 11.03(s, 1H), 10.19(s, 1H), 9.37(s, 2H), 9.07(s, 1H), 8.03(dd, J=7.9, 1.4Hz, 1H ), 7.67-7.56(m, 1H), 7.09-7.00(m, 2H), 6.95(s, 2H), 6.49(d, J=1.9Hz, 1H), 6.27(d, J=1.9Hz, 1H) .
[0041] 13 C NMR (126MHz, dmso) δ175.09, 165....
Embodiment 3
[0042] Embodiment 3: The synthetic route of flavonoid derivative YSW-02 is as follows:
[0043]
[0044]Flavonoid derivatives b (678mg, 1mmol), acetylsalicylic acid c (216mg, 1.2mmol), DMAP (183mg, 1.5mmol) were added to a 100ml one-port bottle, 25ml DMF was added, the system was in suspension, and EDCI (287mg, 1.5mmol), after 1-2 hours, the system was slowly dissolved, stirred overnight at room temperature for 14h, and the reaction was detected by TLC. After the basic reaction of the raw materials was complete, the system was poured into water, and a yellow solid was precipitated, filtered by suction, and purified by column chromatography to obtain Yellow solid 500 mg, yield 59.5%.
[0045] 1 H NMR (500MHz, dmso-d 6 )δ12.12(s, 1H), 8.18(d, J=7.8Hz, 1H), 7.68(d, J=8.6Hz, 1H), 7.50(d, J=7.5Hz, 2H), 7.46-7.20( m, 21H), 7.16(t, J=7.6Hz, 1H), 6.98(s, 1H), 6.61(d, J=1.7Hz, 1H), 5.30(s, 2H), 5.23(s, 2H), 4.99(s, 2H), 4.96(s, 2H), 2.30(s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com