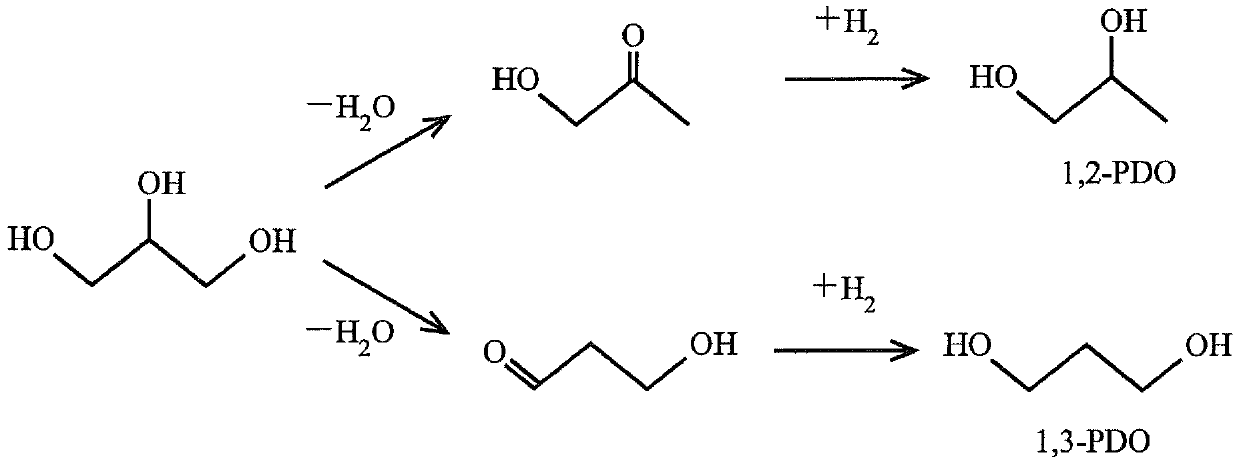
Catalyst for hydrogenolysis of polyhydric alcohol and method for producing 1,3-propanediol using the catalyst
A manufacturing method and catalyst technology, which are applied in catalyst activation/preparation, metal/metal oxide/metal hydroxide catalysts, chemical instruments and methods, etc., can solve the problems of low selectivity, short life, insufficient catalyst activity, etc. Achieving the effect of reducing carbon dioxide emissions, simplifying the manufacturing process, and promoting hydrogenolysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
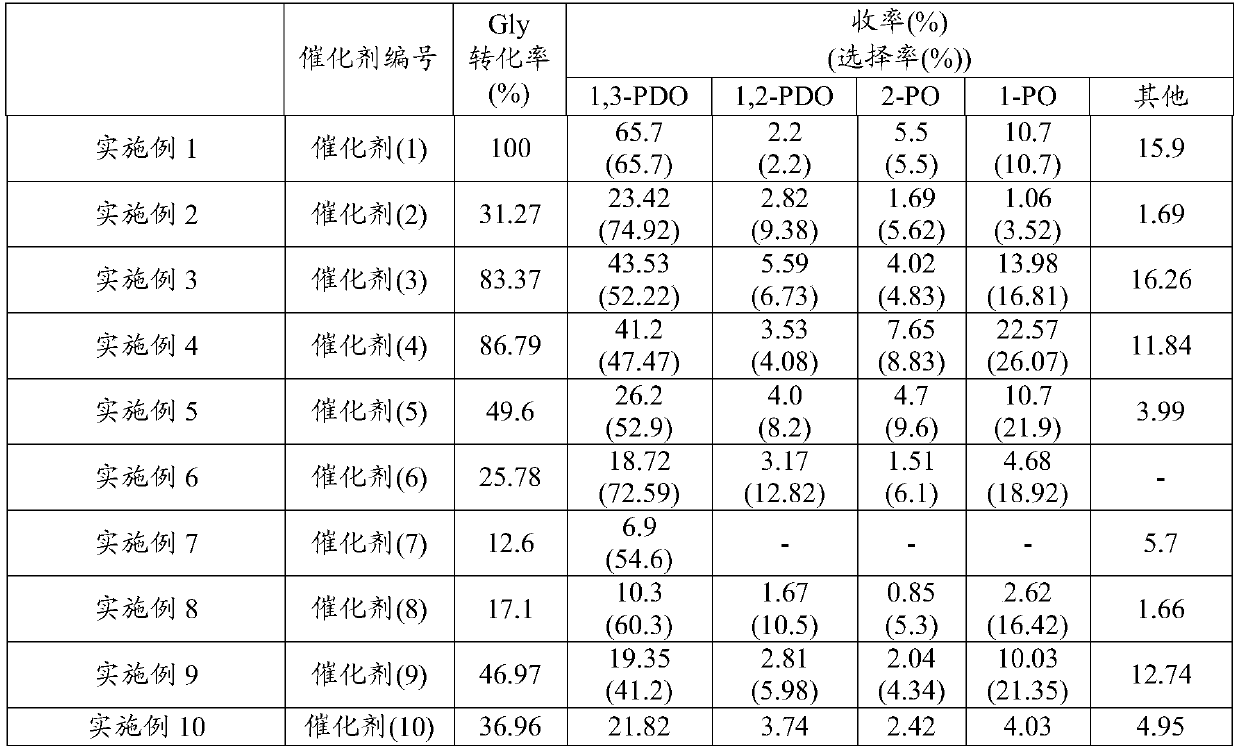
Examples
preparation example 1
[0071] Preparation example 1 (preparation of catalyst)
[0072] Catalysts were prepared by the impregnation method. That is, 20g of boehmite (trade name "Boehmite", manufactured by Wako Pure Chemical Industries, Ltd., average pore diameter: 77nm, specific surface area: 214m 2 / g) into the ammonium paratungstate aqueous solution (1), stirred for 16 hours, distilled off water with an evaporator, dried, and sintered at 800°C for 3 hours, thus obtaining tungsten-loaded boehmite, the ammonium paratungstate aqueous solution (1) is 2g (0.65mmol) (NH 4 ) 10 h 2 (W 2 o 7 ) 6 ·xH 2 O (manufactured by Aldrich) was dissolved in 200 mL of water.
[0073] The boehmite loaded with tungsten obtained by 2g was added in chloroplatinic acid aqueous solution (1), stirred for 12 hours, and described chloroplatinic acid aqueous solution (1) was 2% by weight of chloroplatinic acid aqueous solution (H 2 PtCl 6 : 0.2mmol) 4mL dissolved in 100mL deionized water to obtain.
[0074] After the ...
preparation example 2
[0076] Preparation example 2 (preparation of catalyst)
[0077] Except using chloroplatinic acid aqueous solution (2) instead of chloroplatinic acid aqueous solution (1), obtain catalyst (2) (Pt: 1% by weight, W: 8% by weight) in the same manner as Preparation Example 1, the chloroplatinic acid Aqueous solution (2) is 2% by weight chloroplatinic acid aqueous solution (H 2 PtCl 6 : 0.1mmol) 2mL dissolved in 100mL deionized water.
preparation example 3
[0078] Preparation example 3 (preparation of catalyst)
[0079] Catalyst (3) (Pt: 1% by weight, W: 8% by weight) was obtained in the same manner as in Preparation Example 2, except that the sintering conditions after impregnation were changed from 300°C for 3 hours to 500°C for 3 hours. .
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com