Th detecting method for non-heparin anticoagulant blood sample
A blood sample and detection method technology, applied in the field of Th detection of non-heparin anticoagulated blood samples, can solve the problems of sample type limitation, re-collection, sample waste, etc., and achieve the effect of avoiding repeated collection, avoiding sample waste, and expanding the scope
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
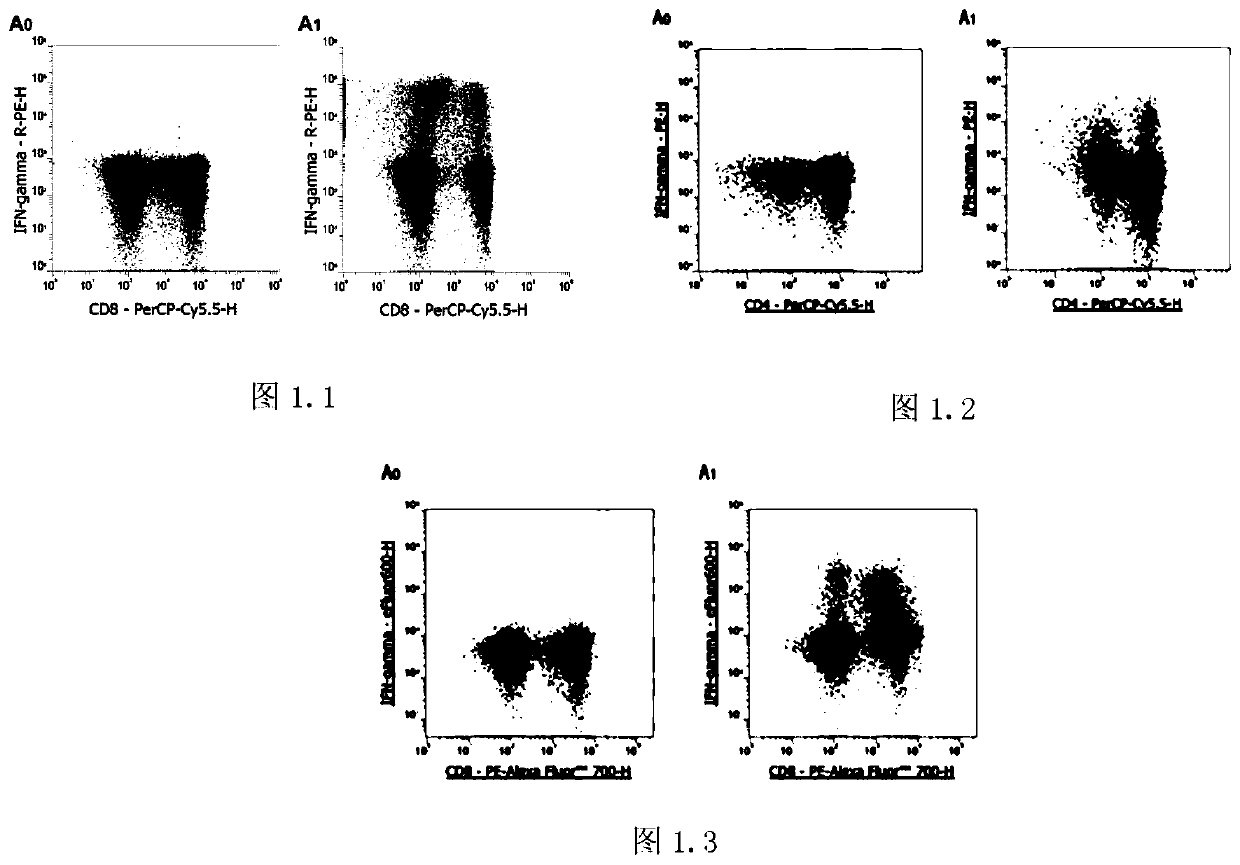
[0031] A Th detection method of a non-heparin anticoagulated blood sample, comprising the following steps:
[0032] Step 1: Separate peripheral blood mononuclear cells (PBMCs) with lymphocyte separation medium, resuspend the pellet with medium containing 10% fetal bovine serum, and make the cell concentration 1×10 7 / ml, take 250μl PBMCs to the flow tube, add 1μl PMA / Ionomycin Mixture (250×) and 1μl BFA / Monensin Mixture (250×), use only PBMCs as a control, mix well, and incubate at 37℃ for 4- 6 hours, take out every 1-2 hours and mix well;
[0033] Step 2: Take 100 μl of cell suspension from the sample tube and the control tube into a new flow tube, add the corresponding flow antibody, shake and mix, and incubate at room temperature in the dark for 15 minutes;
[0034] Step 3: Add 100 μl FIX&PERM Medium A to each tube, vortex to mix, and incubate at room temperature in the dark for 15 minutes;
[0035] Step 4: Dilute 10×Flow Cytometry Staining Buffer to 1× with distilled wat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com