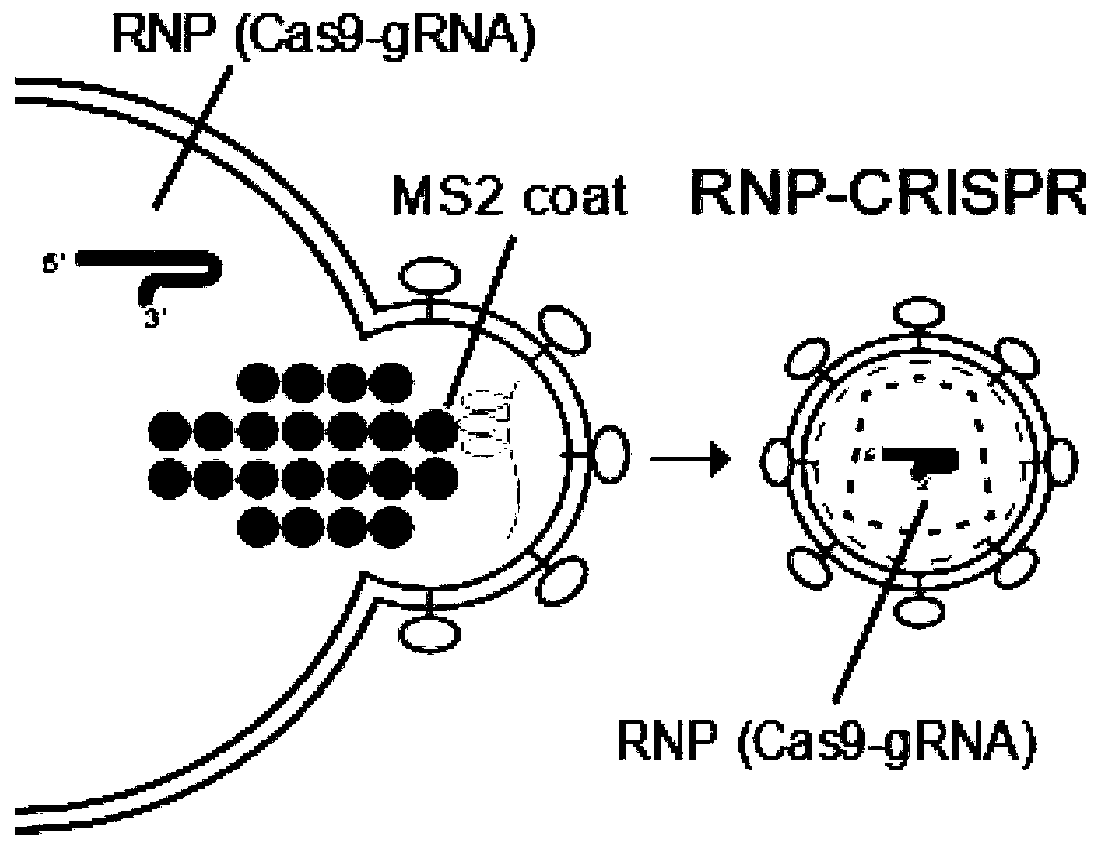
Lentiviral vector for delivering exogenous RNP and preparation method of lentiviral vector
A lentiviral vector and lentiviral technology, applied in the field of lentiviral vector delivery of exogenous RNP and its preparation, can solve the problems of long time and high off-target risk, and achieve the effect of improving application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] This embodiment relates to a lentiviral vector for delivering exogenous RNP and its preparation method; including:
[0033] Step 1: Plasmids expressing membrane proteins (such as plasmids expressing VSV-G), plasmids expressing lentiviral GagPol long-chain proteins containing RNA-binding proteins, plasmids expressing lentiviral GagPol long-chain proteins, and proteins required to form RNP The plasmid and the target RNA expressing the stem-loop structure recognized by the RNA binding protein are co-transfected into virus production cells (such as 293T). in,
[0034] (1) Plasmids expressing membrane proteins include VSV-G, CD4 recognition protein, CD8 recognition protein, RD114, membrane proteins transformed from baboon endogenous retrovirus membrane proteins. In this embodiment, VSV-G is selected. The plasmid expressing VSV-G is the known plasmid pMD2G, and the map of the plasmid expressing VSV-G is shown in SEQ ID NO.1.
[0035] (2) The plasmid expressing the lentivir...
Embodiment 2
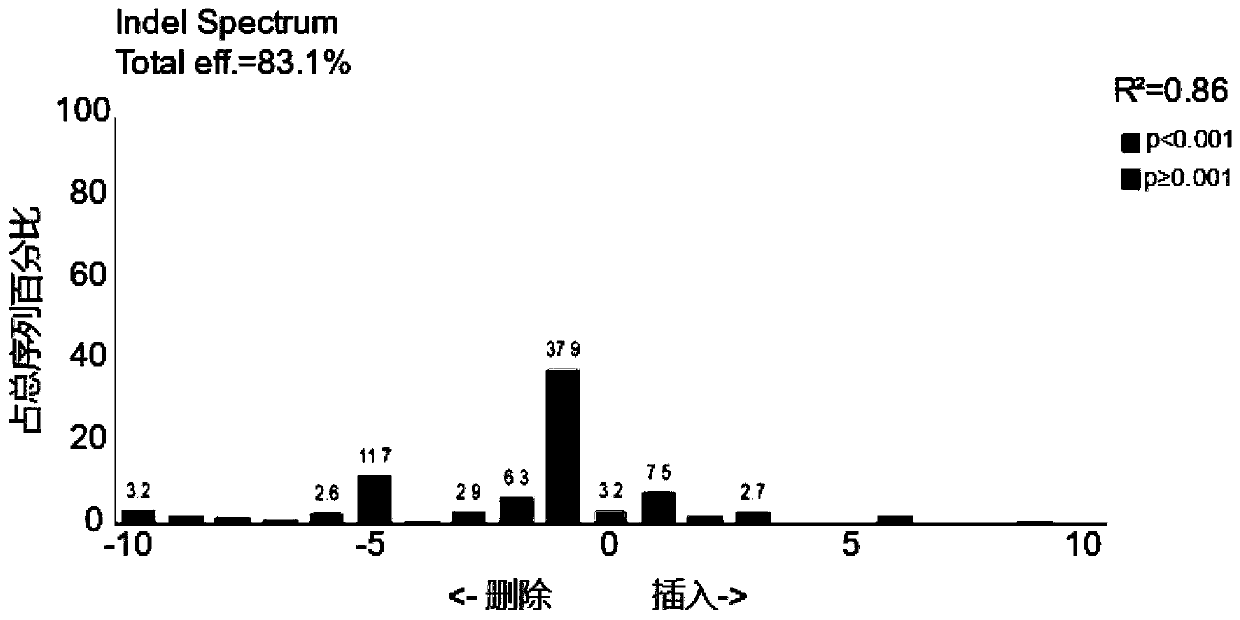
[0078] Example 2. Delivery of RNP (Cas9-gRNA) using lentiviral vectors
[0079] The specific steps are the same as in Example 1, and the exogenous target RNP is a complex of SpCas9 and gRNA. SpCas9 is a nucleic acid cleaving enzyme protein, and the exogenous RNA is selected from the gRNA targeting the AAVS1 site of the human genome, and the sequence is 5'-ggggccactagggcaggat-3' (SEQ ID NO.31); this embodiment is specifically a lentiviral vector Delivery of RNP (Cas9-gRNA) to 293T cells.
[0080] Depend on figure 2 It can be seen that after 293T cells were infected with lentiviral particles carrying RNP (Cas9-gRNA) for 72 hours, the AAVS1 site was amplified by PCR (primer F: 5'-ttcgggtcacctctcactcc-3' (SEQ ID NO.32), primer R: 5' -ggctccatcgtaagcaaacc-3' (SEQ ID NO. 33)) and sequenced with primer F. The gene knockout efficiency of TIDE analysis was as high as 83.1%.
Embodiment 3
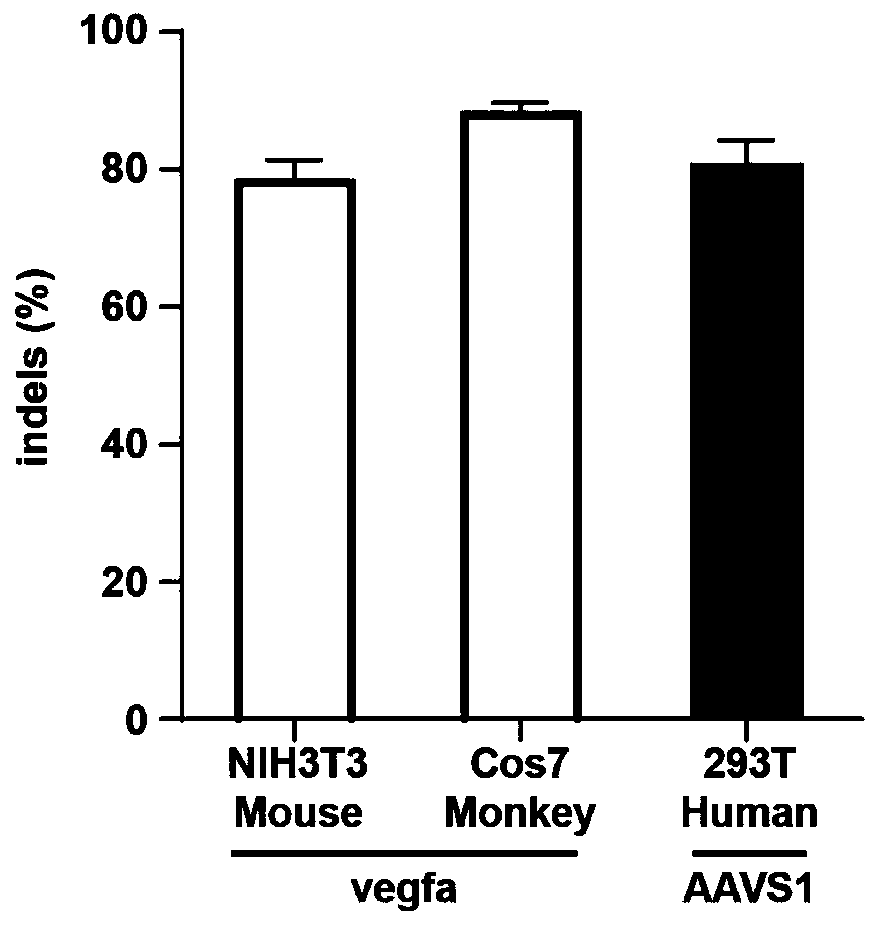
[0081] Example 3. Gene editing of different animal cells using lentiviral vector delivery of RNP (Cas9-gRNA)
[0082] The specific steps are the same as in Example 1, and the exogenous target RNP is a complex of SpCas9 and gRNA. SpCas9 is a nucleic acid cleaving enzyme protein, and the exogenous RNAs were selected from gRNA targeting the vegfa site of the mouse genome, gRNA targeting the vegfa site of the green monkey genome, and gRNA targeting the AAVS1 site of the human genome. The gRNA sequences targeting the vegfa site of the mouse and green monkey genes are both 5'-ctcctggaagatgtccacca-3' (SEQ ID NO.34).
[0083] Depend on image 3 It can be seen that, 72 hours after mouse-derived NIH3T3 cells, green monkey-derived Cos7 cells and human-derived 293T cells were infected with lentiviral particles carrying RNP (Cas9-gRNA), the vegfa or AAVS1 sites were amplified by PCR and sequenced. Primer F: 5'-gatgagtggctgttggcctg-3' (SEQ ID NO.35) for PCR amplification of the vegfa si...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap