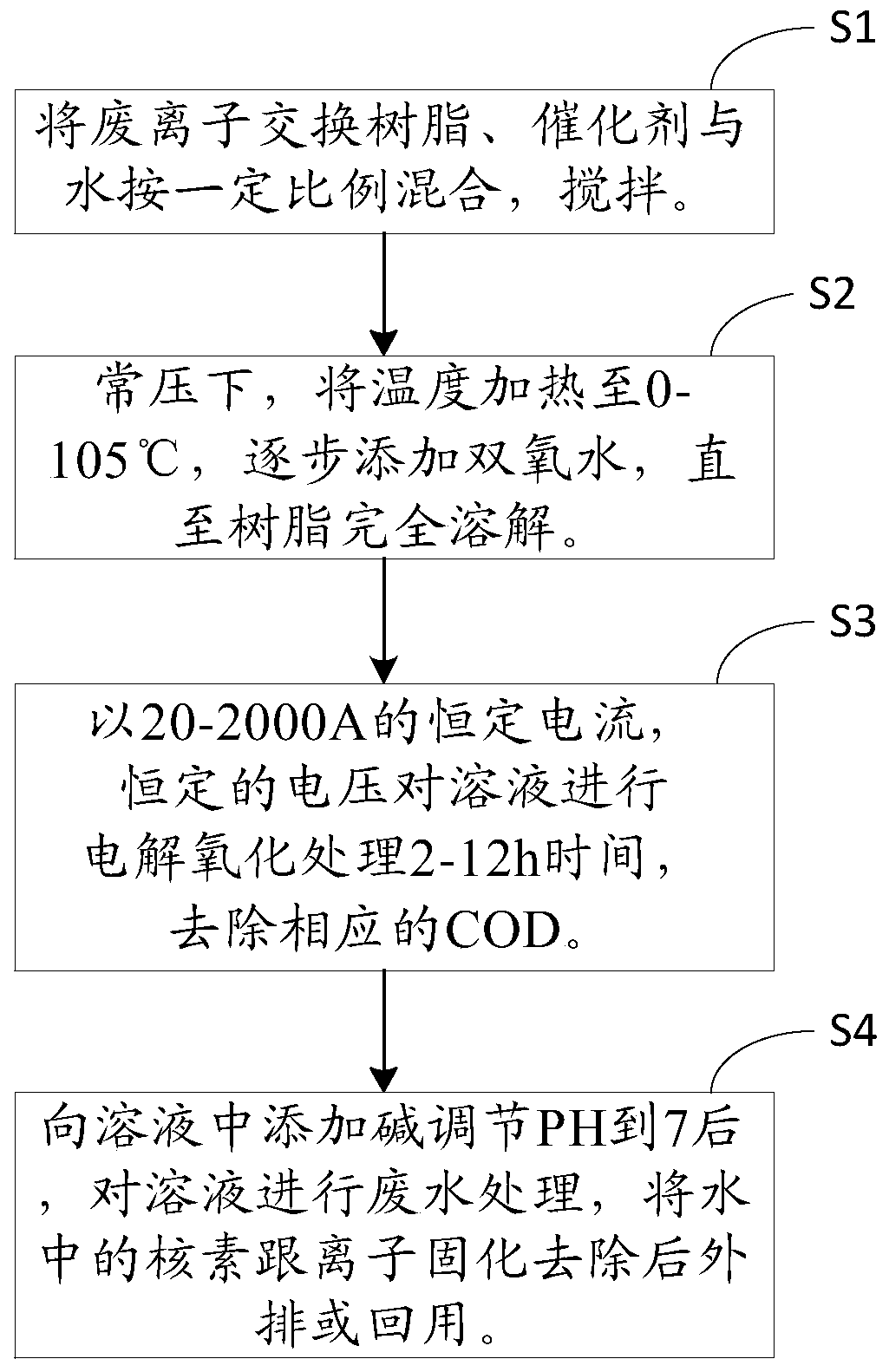
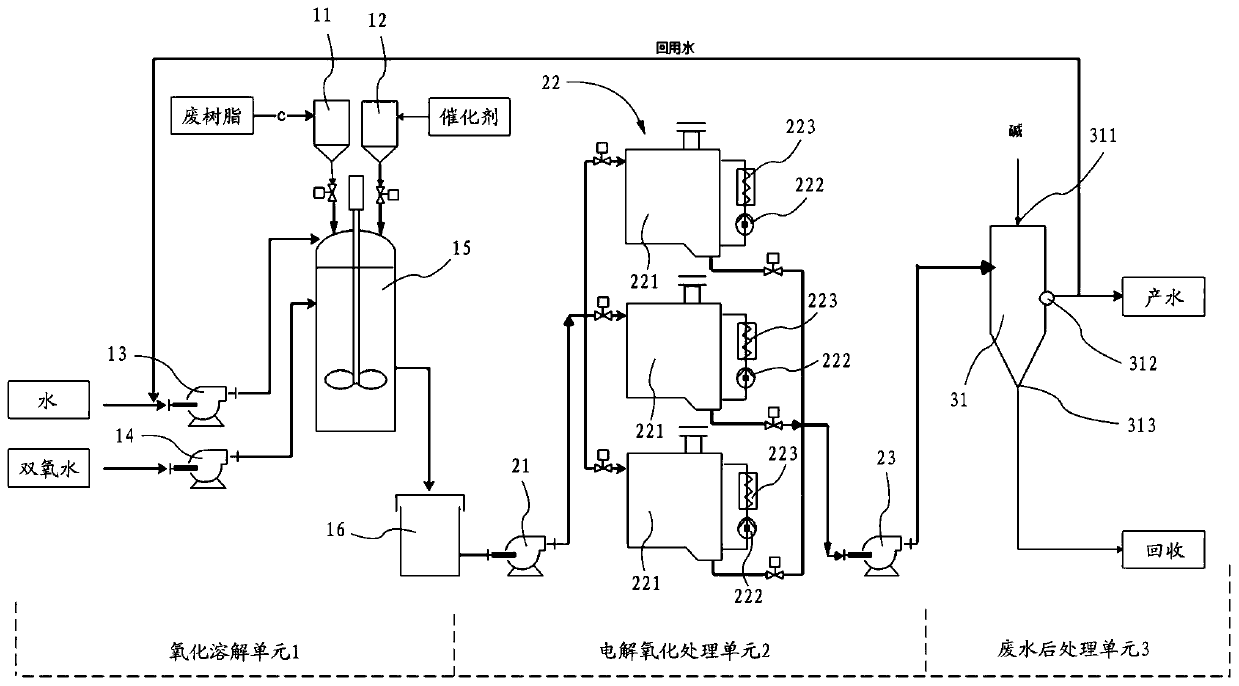
Waste ion exchange resin treatment method and system
A technology for exchanging resins and treatment methods, applied in the direction of electric regeneration, etc., which can solve the problems of high treatment costs, affecting resin oxidation, large amount of catalyst and hydrogen peroxide, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] 33g of ferrous sulfate and 320mL of waste ion exchange resin were added to the oxidation reaction kettle 15, and 9L of deionized water was passed into the water inlet pump 13, and the stirring was started. After the temperature was raised to 70°C, hydrogen peroxide (30% ) 500g, after one hour, the resin has been dissolved in the liquid. This solution is drained into buffer tank 16 . Then enter the electrolytic reaction tank 22 through the electrolytic oxidation liquid inlet pump 21 for further advanced treatment. The current was set to 100A, the initial voltage was 4.5V, and after electrolytic oxidation for 6 hours, the measured COD of the solution was 95mg / L. The solution enters the mixed sedimentation tank 31 through the electrolytic oxidation outlet pump 23, and sodium hydroxide is added to adjust the pH to 7, and then the wastewater is post-treated.
Embodiment 2
[0068]33g of ferrous sulfate and 320mL of waste ion exchange resin were added to the oxidation reaction kettle 15, and 9L of deionized water was passed into the water inlet pump 13, and the stirring was started. After the temperature was raised to 70°C, hydrogen peroxide (30% ) 1000g, after two hours, the resin has been dissolved in the liquid. This solution is drained into buffer tank 16 . Then enter the electrolytic reaction tank 22 through the electrolytic oxidation liquid inlet pump 21 for further advanced treatment. The current was set to 100A, the initial voltage was 4.5V, and after electrolytic oxidation for 5 hours, the measured COD of the solution was 340mg / L. The solution enters the mixed sedimentation tank 31 through the electrolytic oxidation outlet pump 23, and sodium hydroxide is added to adjust the pH to 7, and then the wastewater is post-treated.
Embodiment 3
[0070] 22g of ferrous sulfate and 320mL of waste ion exchange resin were added to the oxidation reaction kettle 15, and 9L of deionized water was passed into the water inlet pump 13, and the stirring was started. After the temperature was raised to 70°C, hydrogen peroxide (30% ) 500g, after one hour, the resin has been dissolved in the liquid. This solution is drained into buffer tank 16 . Then enter the electrolytic reaction tank 22 through the electrolytic oxidation liquid inlet pump 21 for further advanced treatment. The current was set to 100A, the initial voltage was 4.5V, and the COD of the solution was measured to be 425mg / L after electrolytic oxidation for 6h. The solution enters the mixed sedimentation tank 31 through the electrolytic oxidation outlet pump 23, and sodium hydroxide is added to adjust the pH to 7, and then the wastewater is post-treated.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Cod | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap