Methods for aiding in diagnosing and evaluating a mild traumatic brain injury in a human subject using cardiac troponin i and early biomarkers
A biomarker, subject technology, applied in biochemical equipment and methods, biological testing, and microbial determination/examination, etc., can solve the unresearched myocardial injury correlation, disease spectrum bias, unresearched myocardial injury effect, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
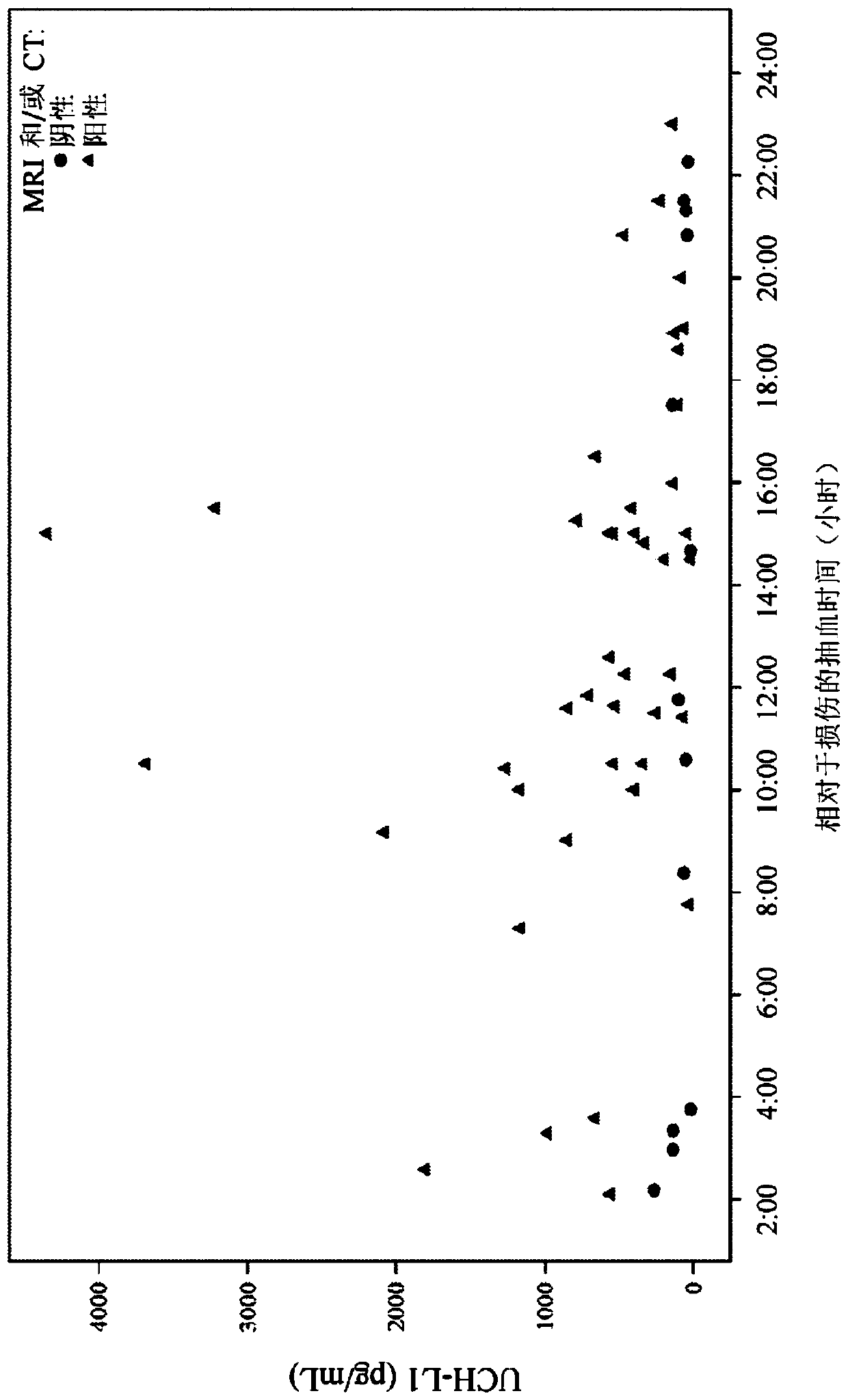
[0514] UCH-L1 assay
[0515] A pair of monoclonal antibodies (such as antibody A) as capture monoclonal antibodies and antibodies B and C as detection monoclonal antibodies were tested. Antibody A is an exemplary anti-UCH-L1 antibody developed in-house at Abbott Laboratories (Abbott Park, IL). Antibodies B and C, which recognize different epitopes of UCH-L1 and enhance detection of antigen in samples, were developed by Banyan Biomarkers (Alachua, Florida). Other antibodies developed in-house at Abbott Laboratories (Abbott Park, IL) also showed or were expected to show similar signal enhancement when used together in various combinations as capture or detection antibodies. The UCH-L1 assay design was evaluated for key performance attributes. Cassette configuration is antibody configuration: Antibody A (capture antibody) / Antibody B+C (detection antibody); reagent conditions: 0.8% solids, 125 μg / mL Fab alkaline phosphatase cluster conjugate; and injection print: UCH-L1 stand...
Embodiment 2
[0517] GFAP assay
[0518] Will The GFAP assay was used in a population study of TBI patients. Use a monoclonal antibody pair such as antibody A as the capture monoclonal antibody and use antibody B as the detection monoclonal antibody. Antibody A and Antibody B are exemplary anti-GFAP antibodies developed in-house at Abbott Laboratories (Abbott Park, IL). The GFAP assay design was evaluated for key performance attributes. The cartridge configuration was antibody configuration: Antibody A (capture antibody) / Antibody B (detection antibody); reagent conditions: 0.8% solids, 250 μg / mL Fab alkaline phosphatase cluster conjugate; and injection print: GFAP specific. The assay time was 10-15 min (with a sample capture time of 7-12 min).
Embodiment 3
[0520] Study 1 - TBI Population
[0521] Study 1 is a large and complex project. Its institutional and public-private partnerships consist of more than 11 clinical sites, seven cores, and a total of nearly 50 partner institutions, companies and charities. An early pilot study based on clinical data from three clinical sites helped refine TBI common data elements and created a prototype for TBI information sharing for Study 1.
[0522] Subject Groups: A total of 2700 to 3000 TBI patients were recruited, averaged into 3 clinical groups divided by clinical care pathways: 1. Patients evaluated in the Emergency Department and discharged (ED); 2. Patients admitted to the hospital but not Patients admitted to ICU (ADM); and 3. Patients admitted to ICU (ICU). An additional 100 patients with extracranial trauma but not TBI were included in each clinical group (n=300) as controls for a total of 3000 patients recruited. This stratification scheme facilitates Comparative Effects Study ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com