A kind of anti-poisoning water-soluble peroxide decomposition catalyst and its preparation method and application
A technology of peroxide decomposition and catalyst, applied in the direction of organic compound/hydride/coordination complex catalyst, physical/chemical process catalyst, organic chemical method, etc. Poison inactivation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
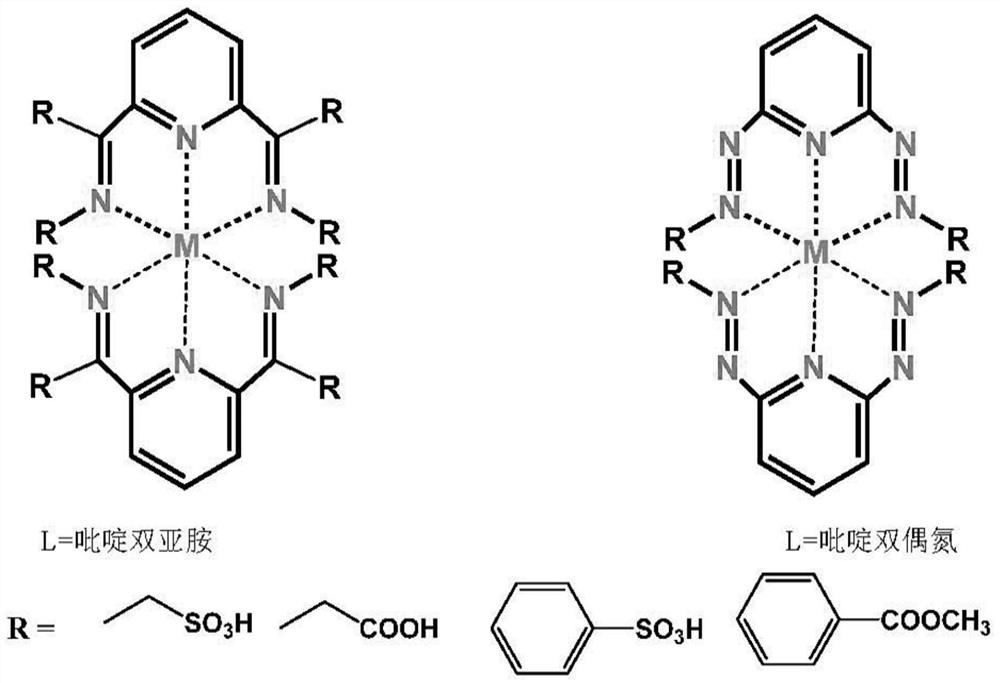
[0042] Synthesis of Mononuclear Iron(Ⅱ) Complex Fe-1
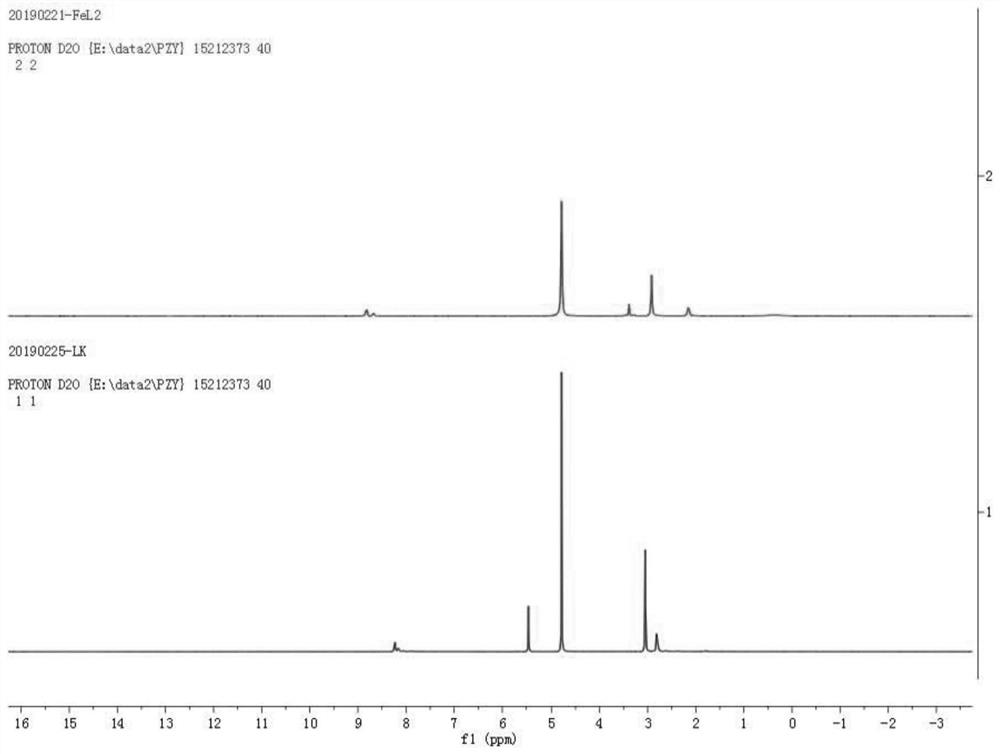
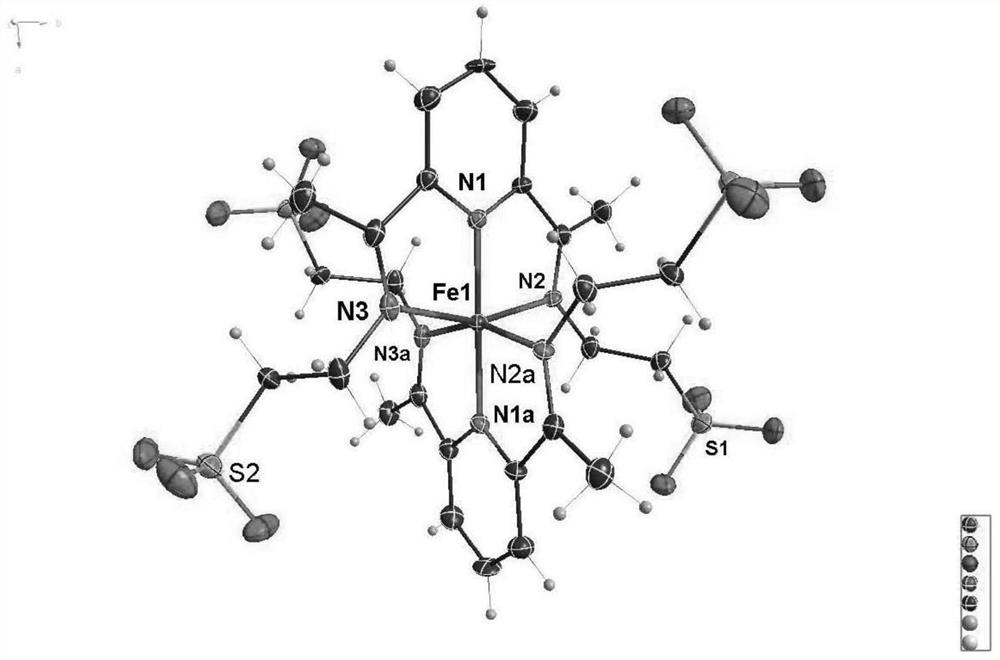
[0043] Pour taurine (500mg, 4mmol), 2,6-diacetylpyridine (326mg, 2mmol), potassium hydroxide (224mg, 4mmol) and methanol (100mL) into a 250mL round bottom flask in turn, and heat to 85°C Reflux for 12 hours. After cooling to room temperature, a milky white solid was obtained by filtration. Denoted as ligand L1; the ligand L1 obtained above was dispersed in (50 ml) methanol solution, and 10 ml of ferrous perchlorate hexahydrate (256 mg, 1 mmol) in methanol solution was slowly added thereto. After stirring for a few minutes, the temperature was raised to 85°C for 2h. Cool to room temperature, filter, concentrate the filtrate to 10 mL by rotary evaporation under reduced pressure, seal it and put it in a refrigerator at -16°C, take it out after standing for 2 hours, and a large number of dark purple microcrystals are precipitated. Denoted as complex Fe-1, its single crystal structure, magnetic properties, electrochemical pr...
Embodiment 2
[0045] Embodiment 2: the synthesis of mononuclear iron (II) complex Fe-2
[0046] Pour beta-alanine (356mg, 4mmol), 2,6-diacetylpyridine (326mg, 2mmol), potassium hydroxide (224mg, 4mmol) and methanol (100mL) into a 250mL round bottom flask in turn, and heat to Reflux reaction at 85°C for 12 hours, the solution gradually changed from colorless to orange yellow. After cooling to room temperature, the solvent was removed by rotary evaporation under reduced pressure, and the product was washed 3 times with cold methanol to obtain a yellow solid. Denoted as ligand L2; ligand L2 was dissolved in (50ml) methanol solution, and 10ml of ferrous perchlorate hexahydrate (256mg, 1mol) in methanol solution was slowly added thereto. After stirring for a few minutes, the temperature was raised to 85°C for 2h. Cool to room temperature, filter, concentrate the filtrate to 10 mL by rotary evaporation under reduced pressure, seal it and put it in a refrigerator at -16°C, and take it out after ...
Embodiment 3
[0048] Embodiment 3: the synthesis of mononuclear iron (II) complex Fe-3
[0049] Pour 4-aminobenzenesulfonic acid (692mg, 4mmol), 2,6-diacetylpyridine (326mg, 2mmol), potassium hydroxide (224mg, 4mmol) and methanol (100mL) into a 250mL round bottom flask in turn, and heat to Reflux at 85°C for 12 hours. After cooling to room temperature, a milky white solid was obtained by filtration. Denoted as ligand L3; the ligand L3 obtained above was dispersed in (50 ml) methanol solution, and 10 ml of ferrous perchlorate hexahydrate (256 mg, 1 mol) in methanol solution was slowly added thereto. After stirring for a few minutes, the temperature was raised to 85°C for 2h. Cool to room temperature, filter, concentrate the filtrate to 10 mL by rotary evaporation under reduced pressure, seal it and put it in a refrigerator at -16°C, take it out after standing for 2 hours, and a large number of dark purple microcrystals are precipitated. Recorded as complex Fe-3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com