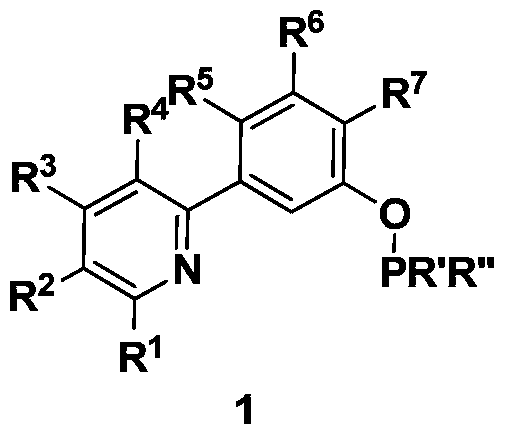
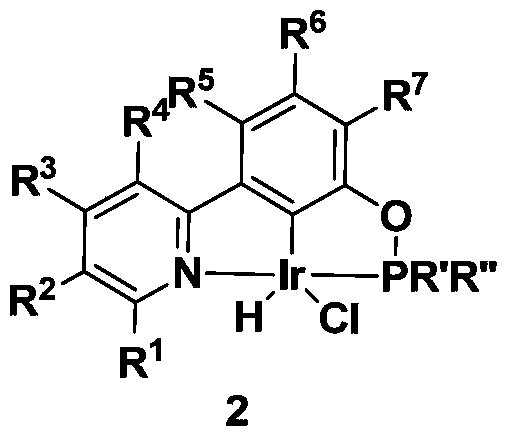
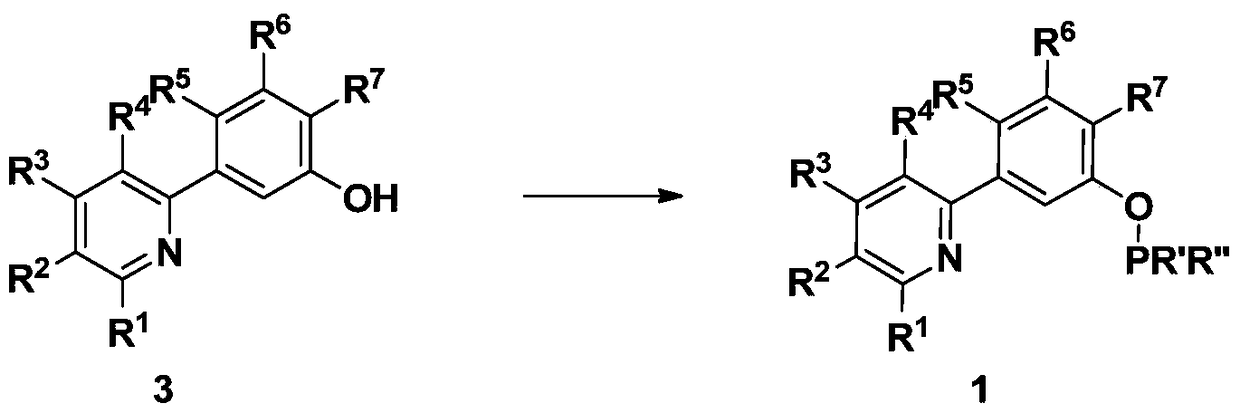
ncp ligand, its iridium complex, synthesis method, intermediate and application
A technology of iridium complexes and ligands, applied in the field of NCP ligands, can solve the problems of low catalytic efficiency, harsh reaction conditions, poor selectivity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0156] Embodiment 1: preparation of the present invention H NCP ligand
[0157]
[0158] Add 3-methoxyphenylboronic acid (5.0g, 32.8mmol), K 2 CO 3 (9.4g, 68.0mmol), then set up a reflux device, pump and change the gas 3 times, add 2-bromopyridine (4.4g, 27.8mmol), solvent ethylene glycol dimethyl ether (50mL) and Distilled water (33 mL). Then the system was bubbled with a long needle for 30 min, and tetrakistriphenylphosphopalladium (1.3 g, 1.1 mmol) was added. The system was heated to reflux for 12h in an argon atmosphere. After the reaction, the system was cooled to room temperature, extracted with ethyl acetate (30mL×3), the collected organic phase was backwashed with saturated brine, dried over anhydrous sodium sulfate, concentrated by filtration, and purified by column chromatography (petroleum ether: ethyl acetate = 1:20), to obtain 3.2g of colorless oil 2-(3-methoxyphenyl)pyridine, yield 61%.
[0159] Add 2-(3-methoxyphenyl)pyridine (3.2g, 17.3mmol) and 40% aq...
Embodiment 2
[0163] Embodiment 2: preparation of the present invention Me NCP ligand
[0164]
[0165] Add 3-methoxyphenylboronic acid (5.0 g, 32.6 mmol), K 2 CO 3 (9.8g, 71mmol), then set up the reflux device, pump and change the gas 3 times, add 2-bromo-6-methylpyridine (5.1g, 29.8mmol) and solvent ethylene glycol dimethyl ether under argon protection gas (60mL) and distilled water (38mL). Then the system was bubbled with a long needle for 30 min, and tetrakistriphenylphosphopalladium (1.7 g, 1.5 mmol) was added. The system was heated to reflux for 12h in an argon atmosphere. After the reaction, the system was cooled to room temperature, extracted with ethyl acetate (30mL×3), the collected organic phase was backwashed with saturated brine, dried over anhydrous sodium sulfate, concentrated by filtration, and purified by column chromatography (petroleum ether: ethyl acetate = 1:50), and 3.0 g of colorless oil 2-(3-methoxyphenyl)-6-picoline was obtained with a yield of 51%.
[0166...
Embodiment 3
[0171] Embodiment 3: preparation of the present invention tBu NCP ligand
[0172]
[0173] Add 3-methoxyphenylboronic acid (3.1g, 20.0mmol), K 2 CO 3 (5.8g, 42.0mmol), then set up the reflux device, pump and change the gas 3 times, add 2-bromo-6-tert-butylpyridine (3.6g, 16.8mmol) and solvent ethylene glycol di methyl ether (30 mL) and distilled water (20 mL). Then the system was bubbled with a long needle for 30 minutes and tetrakistriphenylphosphopalladium (1.0 g, 0.9 mmol) was added. The system was heated to reflux for 12h in an argon atmosphere. After the reaction, the system was cooled to room temperature, extracted with ethyl acetate (30mL×3), the collected organic phase was backwashed with saturated brine, dried over anhydrous sodium sulfate, concentrated by filtration, and purified by column chromatography (petroleum ether: ethyl acetate = 1:100), to obtain 2.7g of colorless oil 2-(3-methoxyphenyl)-6-tert-butylpyridine, yield 67%.
[0174] 1 H NMR (400MHz, CD...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com