Preparation method of butyl rubber
A technology of butyl rubber and dialkyl, which is applied in the field of preparing butyl rubber by rare earth catalyst, can solve the problems of affecting product quality, difficult to meet the economical production of industrialized production, and high metal residues in polymerization activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
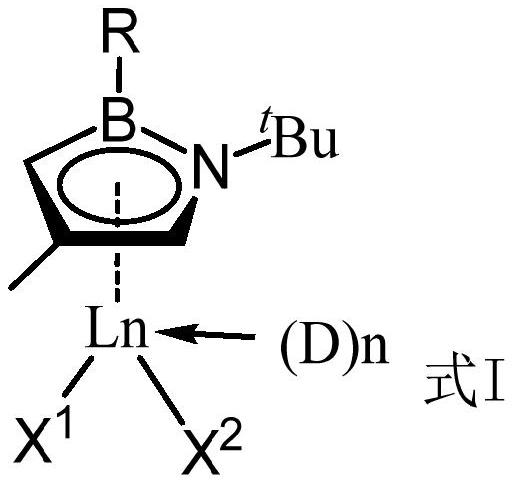
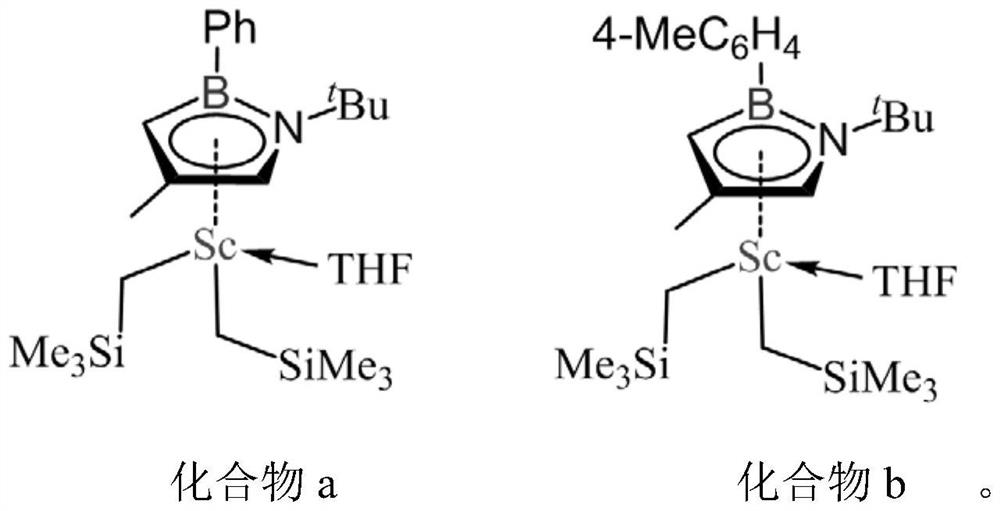
[0032] Preparation process of boron nitrogen heterocyclic rare earth compounds a and b
[0033] Under nitrogen or argon atmosphere, react 2 mol of borazine ligand 1 with 2 mol of n-butyllithium in 10 mL of tetrahydrofuran solution at -40°C for 1 hour, then slowly add the solution dropwise to 2 mol of ScCl at room temperature 3 tetrahydrofuran (10mL) suspension, then reacted at room temperature for 1 hour, slowly added 4 mol of trimethylsilyl methylene lithium to the above solution, reacted at room temperature for about 1 hour, vacuumed all the solvents, and used 20mL of toluene was extracted, concentrated, and recrystallized at -35°C to obtain borazine heterocyclic rare earth compound a.
[0034] In the same way, ligand 2 was used to prepare borazine heterocyclic rare earth compound b.
[0035] Borazine Ligand Ligand
[0036]
Embodiment 1
[0038] Preparation of isobutylene homopolymer
[0039] Under nitrogen protection, 9.4 grams of 30 wt% isobutylene toluene solution was added to a 50 ml reaction kettle at -30°C, and then 10 micromoles of compound a and 10 micromoles of organoboron salt [Ph 3 C][B(C 6 f 5 ) 4 ] composition in toluene (1 ml) and polymerization was initiated. After stirring and reacting at -30°C for 60 minutes, the polymerization solution was poured into a container containing 100 ml of ethanol to terminate the reaction, and the polyisobutene after sedimentation was dried to constant weight in a vacuum oven at 60°C, and the monomer conversion rate was 85. %. The number average molecular weight M of gained polyisobutylene is tested by gel chromatography n =6.14×10 4 ,M w / M n =2.21; glass transition temperature T g = -71°C.
Embodiment 2
[0041] Preparation of isobutylene homopolymer
[0042] Under nitrogen protection, 11.2 grams of 30 wt% isobutylene toluene solution was added to a 25 ml reaction kettle at -20°C, and then 10 micromoles of compound a and 10 micromoles of organoboron salt [Ph 3 C][B(C 6 f 5 ) 4 ] composition in toluene (1 ml) and polymerization was initiated. After stirring and reacting at -20°C for 60 minutes, the polymerization solution was poured into a container containing 100 ml of ethanol to terminate the reaction, and the polyisobutene after sedimentation was dried in a vacuum oven at 60°C to constant weight, and the monomer conversion rate was 81. %. The number average molecular weight M of gained polyisobutylene is tested by gel chromatography n =3.24×10 4 ,M w / M n =2.04; glass transition temperature T g = -69°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com