A kind of fluorescent compound of β-carbolinium salt for mitochondrial targeting and photodynamic therapy and its preparation method and application
A fluorescent compound and photodynamic therapy technology, applied in the field of biomedicine, can solve the problem of lack of integrated strategy of tumor cell diagnosis and treatment, and achieve the effect of low dark toxicity, broad application and good mitochondrial targeting.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
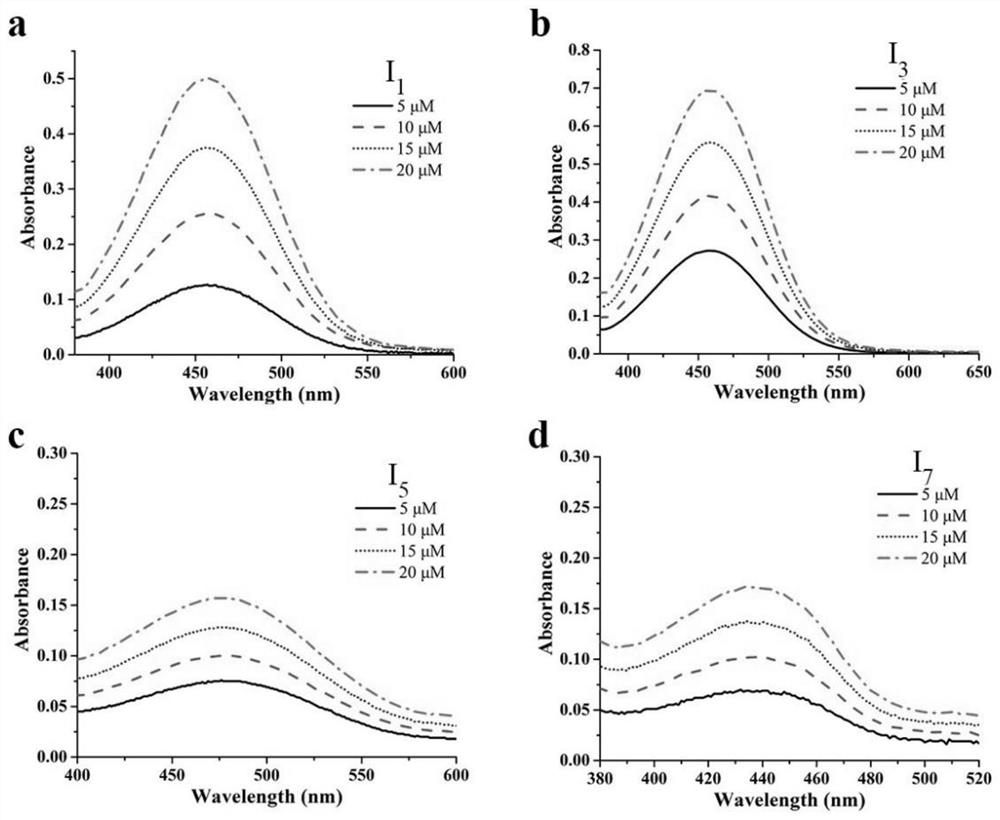
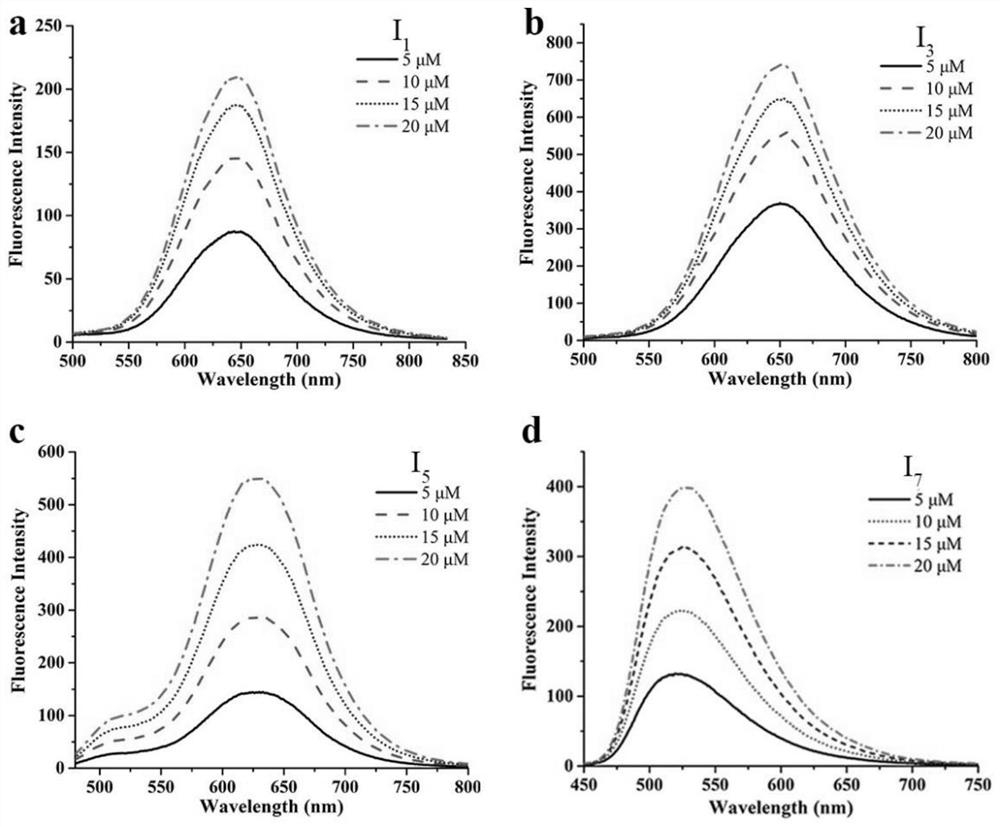
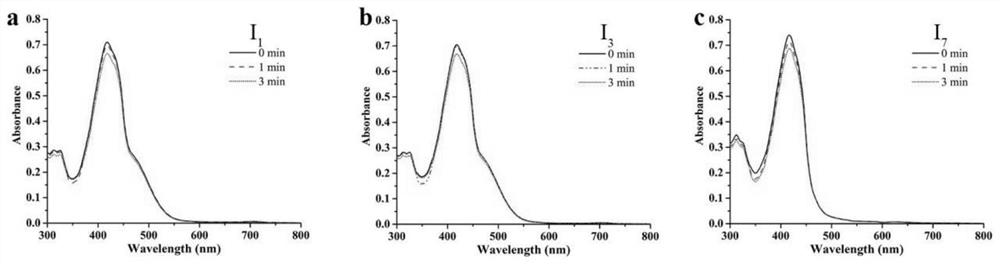
[0039] Example 1: (E)-4-(2-(1,9-Dimethyl-9H-pyrido[3,4-b]indol-3-yl)vinyl)-1-methylquinoline -1-Iodine salt (I 1 ) preparation;
[0040]
[0041] 1,9-Dimethyl-9H-pyrido[3,4-b]indole-3-carbaldehyde (2.24g, 10mmol) and 1,4-dimethylquinoline-1-iodide (2.85g , 10mmol) into a single-necked bottle, add 5ml of absolute ethanol, then add 1 drop of piperidine, reflux overnight, TLC monitors the reaction until complete, the reaction solution is cooled and filtered, and recrystallized and purified again to obtain a red solid (I 1 )4.2g, the yield was 85.7%. (I 1 ) The spectrum data is: ESI-MS(m / z):492[M+H] + ; 1 HNMR(d 6 -DMSO, 400MHz): δ9.29(d, J=6.5Hz, 1H, Ar-H), 8.86(d, J=8.5Hz, 1H, Ar-H), 8.59(s, 1H, Ar-H) ,8.56–8.37(m,3H,Ar-H,CH),8.34–8.20(m,3H,Ar-H,CH),8.12–8.04(m,1H,Ar-H),7.77(d,J= 8.4Hz,1H,Ar-H),7.72–7.63 (m,1H,Ar-H),7.38–7.34(m,1H,Ar-H),4.50(s,3H,CH 3 ),4.20(s,3H,CH 3 ),3.15(s,3H,CH 3 ).
Embodiment 2
[0042] Example 2: (E)-4-(2-(1,9-dimethyl-9H-pyrido[3,4-b]indol-3-yl)ethenyl)-1-(butan-3 -Alkyn-1-yl)quinoline-1-methanesulfonate (I 2 ) preparation;
[0043] With reference to (I in embodiment 1 1 ) synthetic method, 1,4-dimethylquinoline-1-iodine in the method is replaced by 4-methyl-1-(but-3-yn-1-yl) quinoline-1-methanesulfonate salt, and finally a reddish-brown solid (I 2 )3.9g, the yield was 79.6%. (I 2 ) spectrogram data is: ESI-MS (m / z): 498[M+H] + ; 1 HNMR(d 6 -DMSO, 400MHz): δ9.31(d, J=6.6Hz, 1H, Ar-H), 8.89 (d, J=8.6Hz, 1H, Ar-H), 8.58(s, 1H, Ar-H) ,8.58–8.41(m,3H,Ar-H,CH),8.37–8.25(m,3H,Ar-H,CH),8.15–8.07(m,1H,Ar-H),7.80(d,J= 8.4Hz,1H,Ar-H),7.71–7.64(m,1H,Ar-H), 7.42–7.34(m,1H,Ar-H),4.69–4.61(m,2H,CH 2 ),4.26(s,3H,CH 3 ),3.17(s,3H,CH 3 ), 2.14(s, 1H, CH), 2.10–2.04(m, 2H, CH 2 ).
Embodiment 3
[0044] Example 3: (E)-4-(2-(9-(2-ethyl)-1-methyl-9H-pyrido[3,4-b]indol-3-yl)ethenyl)- 1-methylquinoline-1-iodide salt (I 3 ) preparation;
[0045] With reference to (I in embodiment 1 1 ) synthetic method, the 1,9-dimethyl-9H-pyrido[ 3,4-b] indole-3-carbaldehyde, finally obtained red solid (I 3 )4.7g, the yield is 80.5%. (I 3 ) spectrum data is: ESI-MS (m / z): 506[M+H] + ; 1 HNMR(d 6 -DMSO,400MHz):δ9.28(s,1H,Ar-H), 8.85(d,J=8.4Hz,1H,Ar-H),8.57(s,1H,Ar-H),8.54–8.39( m,3H,Ar-H,CH),8.33–8.23(m,3H,Ar-H,CH),8.13–8.05(m,1H,Ar-H),7.73(d,J=8.4Hz,1H, Ar-H),7.70–7.62(m,1H,Ar-H),7.32–7.29(m,1H,Ar-H),4.53(s,3H,CH 3 ),4.25–4.17(m,2H,CH 2 ),3.16(s,3H,CH 3 ), 2.72–2.59 (m,3H,CH 3 ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| emission peak | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com