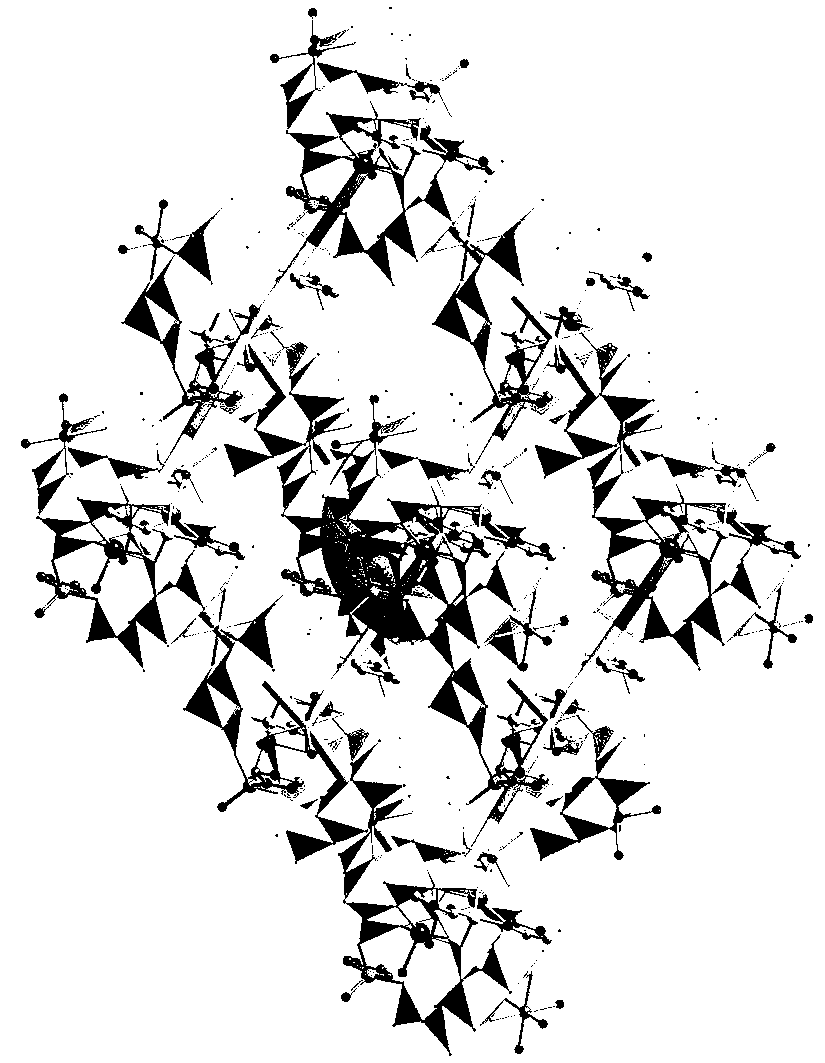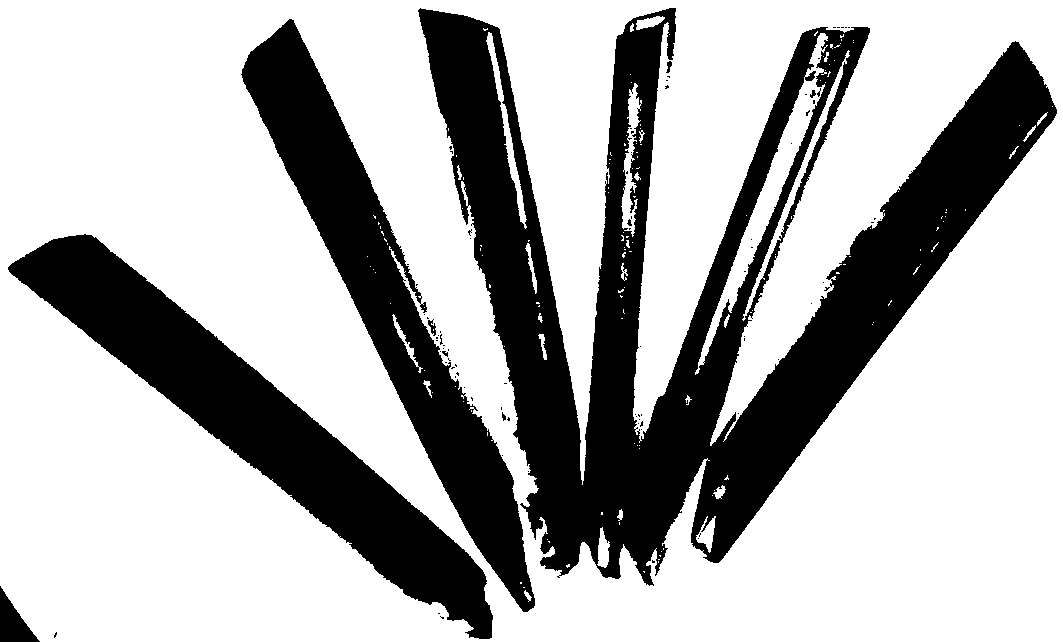Preparation method of polyacid based tubular monocrystal microreactor
A micro-reactor, tubular technology, applied in the field of polyacid-based tubular single-crystal micro-reactor preparation, can solve the problem of lack of crystal structure and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
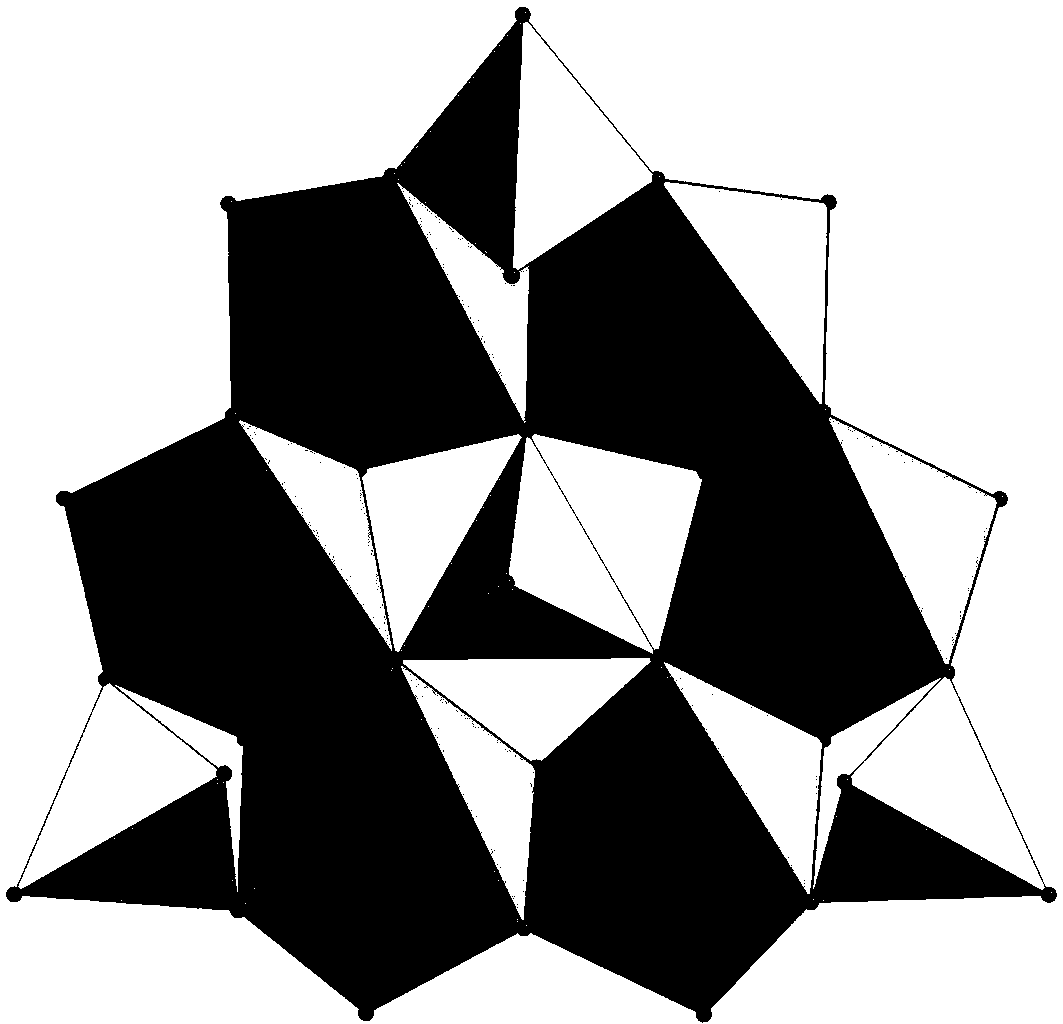
[0020] A polyacid-based tubular single-crystal microreactor whose chemical composition is: Mn[Zn(im)] 2 {[Na(H 2 O)] 2 [Mn(H 2 O) 2 ][Zn(im) 2 ][P 4 Mo 6 o 31 h 6 ] 2} 8H 2 O.
[0021] Preparation method: Add molybdate and phosphorous acid in distilled water at a molar ratio of 5:1, and use hydrochloric acid (6 mol L -1 ) to adjust the pH of the mixture between 5 and stir for 20 minutes. Imidazole (im), manganese chloride, and zinc acetate were sequentially added to the mixture and stirred for 20 minutes. Then put it into a high-pressure reaction kettle, raise the temperature to 170°C, keep it warm for 60 hours, then lower it to room temperature at a speed of 1-10°C / min, and filter to obtain a polyacid-based tubular single crystal microreactor.
Embodiment 2
[0023] A polyacid-based tubular single-crystal microreactor whose chemical composition is: Mn[Zn(im)] 2 {[Na(H 2 O)] 2 [Mn(H 2 O) 2 ][Zn(im) 2 ][P 4 Mo 6 o 31 h 6 ] 2} 8H 2 O.
[0024] Preparation method: Add molybdate and phosphorous acid in distilled water at a molar ratio of 5:1, and use hydrochloric acid (6 mol L -1 ) to adjust the pH of the mixture to 3 and stir for 30 minutes. Imidazole (im), manganese chloride, and zinc acetate were sequentially added to the mixture and stirred for 30 minutes. Then put it into a high-pressure reaction kettle, raise the temperature to 160°C, keep it warm for 48 hours, then lower it to room temperature at a speed of 1-10°C / min, and filter to obtain a polyacid-based tubular single crystal microreactor.
Embodiment 3
[0026] A polyacid-based tubular single-crystal microreactor whose chemical composition is: Mn[Zn(im)] 2 {[Na(H 2 O)] 2 [Mn(H 2 O) 2 ][Zn(im) 2 ][P 4 Mo 6 o 31 h 6 ] 2} 8H 2 O.
[0027] Preparation method: add molybdate and phosphorous acid in distilled water at a molar ratio of 5:1, and use hydrochloric acid (6 mol L -1) to adjust the pH of the mixture at 6 and stir for 30 minutes. Imidazole (im), manganese chloride, and zinc acetate were sequentially added to the mixture and stirred for 30 minutes. Then put it into a high-pressure reaction kettle, raise the temperature to 180°C, keep it warm for 72 hours, then lower it to room temperature at a speed of 1-10°C / min, and filter to obtain a polyacid-based tubular single crystal microreactor.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com