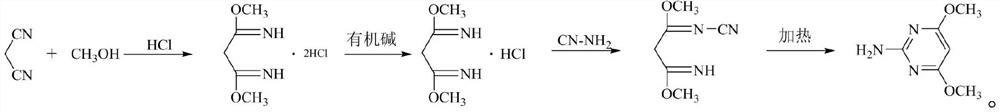
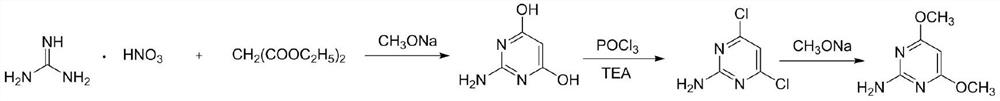
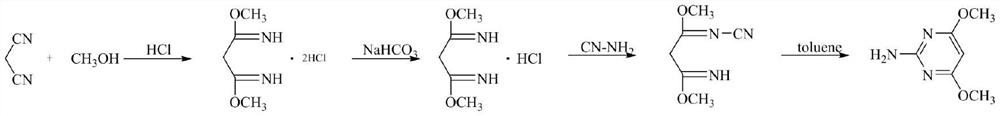
A kind of preparation method of 2-amino-4,6-dimethoxypyrimidine
A technology of dimethoxypyrimidine and amino group is applied in the field of synthesis of important intermediates of pesticides. , The effect of reducing equipment investment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Add 500mL of anhydrous toluene into the 1000mL reactor, cool down to 0-5°C, open the hydrogen chloride gas vent valve and ventilate the reactor for 20 minutes, when a large amount of gas emerges from the exhaust gas and toluene no longer absorbs hydrogen chloride, stop the ventilation; slowly drip Add the methanol (65g, 2.0mol) solution of malononitrile (66g, 1.0mol), control the temperature at 0~5 ℃, and complete the dropwise addition in about 30 minutes, control the reaction temperature at 5~10 ℃ and react for 1 hour after the addition The hydrochloric acid gas dissolved in toluene is reacted, otherwise the exotherm is obvious during ventilation, and it will become impurities when the temperature rises to more than 30 degrees), and then continue to pass hydrogen chloride gas for about 20 hours, the remaining 0.5% of the raw materials and intermediates in the HPLC are controlled and the reaction ends. , close the ventilation valve, open the vacuum valve, and degas the e...
Embodiment 2
[0036] Add 600mL of anhydrous xylene to the 1000mL reactor, cool down to 0-5°C, open the hydrogen chloride gas vent valve to ventilate the reactor for 20 minutes, stop ventilating when a large amount of gas emerges from the tail gas and no longer absorb hydrogen chloride; slowly drip Add the methanol (96g, 3.0mol) solution of malononitrile (66g, 1.0mol), control temperature 5~10 ℃ about 30 minutes and complete dropwise addition, control reaction temperature 8~15 ℃ to react for 1 hour after feeding, then continue to pass through Hydrogen chloride gas is about 18 hours, and the reaction is finished with less than 0.5% of the remaining raw materials and intermediates in the HPLC; close the ventilation valve, open the vacuum valve, negative pressure -0.095Mpa degassing and extract excess hydrogen chloride gas in the system.
[0037] Then the temperature was raised to 10~20°C, triethylamine (102g, 1.0mol) was added dropwise to the reaction system, the pH of the system was adjusted t...
Embodiment 3
[0041] Add 600mL of anhydrous chlorobenzene to the 1000mL reactor, cool down to 0-5°C, open the hydrogen chloride gas vent valve to ventilate the reactor for 20 minutes, and stop ventilating when a large amount of gas emerges from the tail gas and chlorobenzene no longer absorbs hydrogen chloride; The methanol (70g, 2.19mol) solution of malononitrile (66g, 1.0mol) was slowly added dropwise, the temperature was controlled at 0~10°C for about 30 minutes and the dropwise addition was completed. Continue to pass the hydrogen chloride gas for about 24 hours, and the reaction ends below 0.5% of the remaining raw materials and intermediates in the HPLC; close the ventilation valve, slowly open the vacuum valve, and degas the excess hydrogen chloride gas in the system at a negative pressure of -0.095Mpa.
[0042] Then the temperature was raised to 10~20°C, triethylamine (150g, 1.49mol) was added dropwise to the reaction system, the pH of the system was adjusted to 8 with a pH meter, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com