Biomarker for monitoring or diagnosing onset of liver cancer in high-risk group for liver cancer and use thereof
A biomarker and marker technology, which can be used in disease diagnosis, biomaterial analysis, instruments, etc., and can solve problems such as difficulty in use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0126] Example 1: Clinical Specimen Information Used in This Application
[0127] Used to obtain Seoul Asan Hospital Serum samples from a total of 440 patients who were approved by the Clinical Trial Review Board (IRB Approval No. 2015-1156) in high-risk groups of liver cancer (hepatitis B and cirrhosis) and diagnosed patients with liver cancer. Immediately after blood samples were collected from each patient, serum was collected by centrifugation at 4° C. and 3000 rpm for 10 minutes. Serum was dispensed in 50 μl units and stored at 80° C. until used for analysis.
[0128] Patients with high-risk groups of liver cancer provided by Seoul Asan Hospital and patients diagnosed with liver cancer are all patients over 20 years old who have been diagnosed with good liver function (Child-Pugh class A). Very early liver cancer patients with tumors less than 5cm found in MRI) and histological examination. As for the samples of patients in high-risk groups of liver cancer, samples of...
Embodiment 2
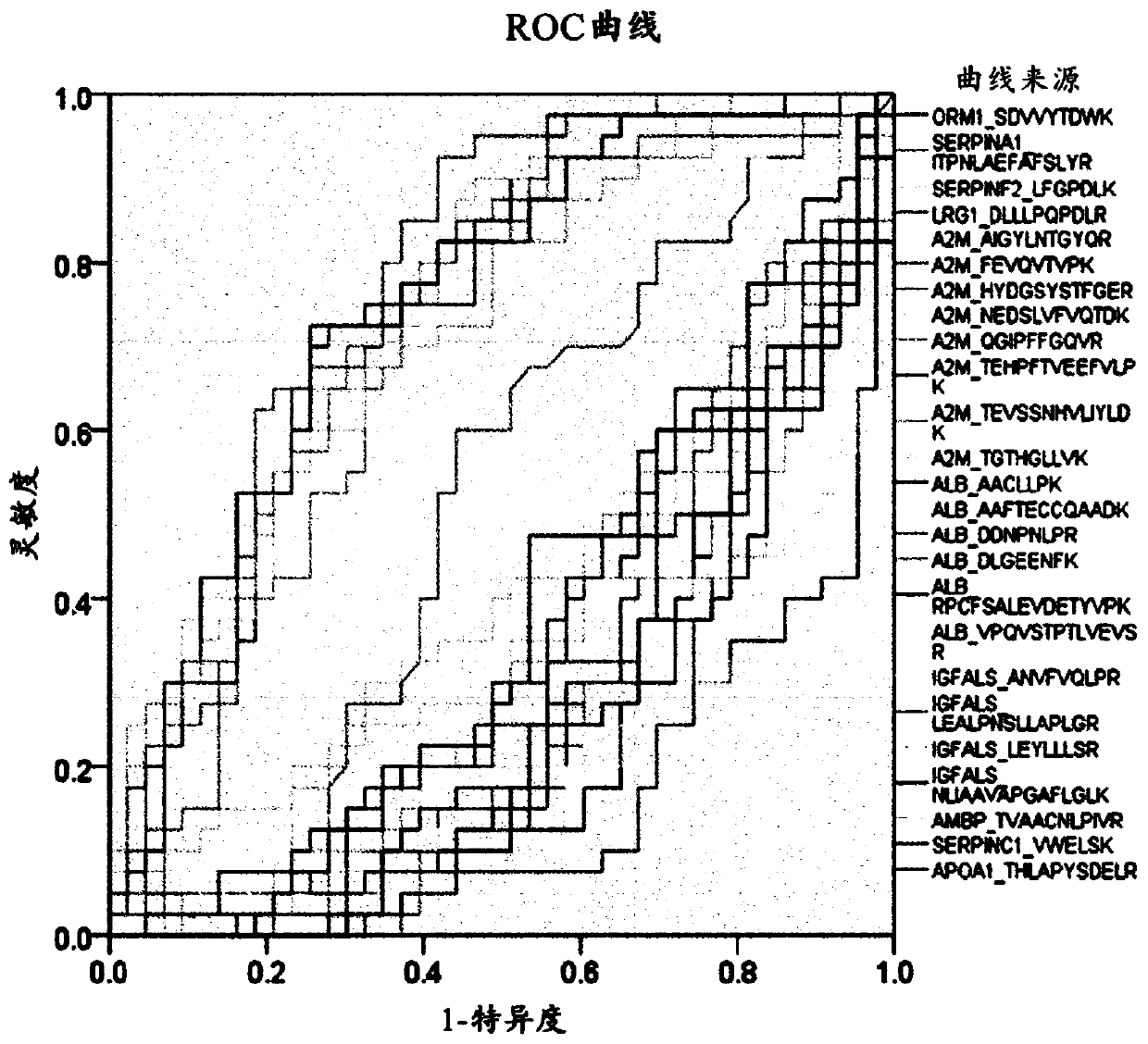
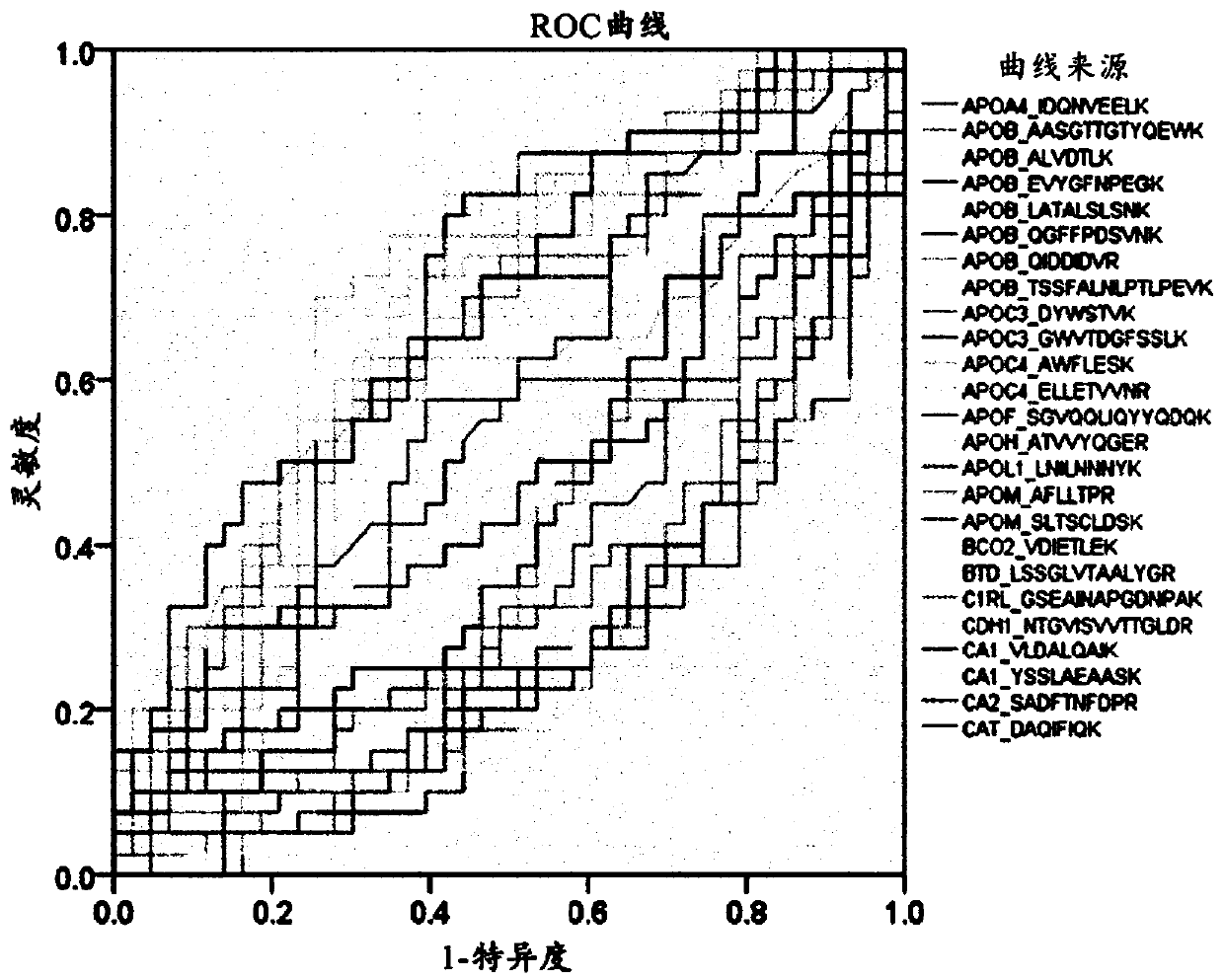
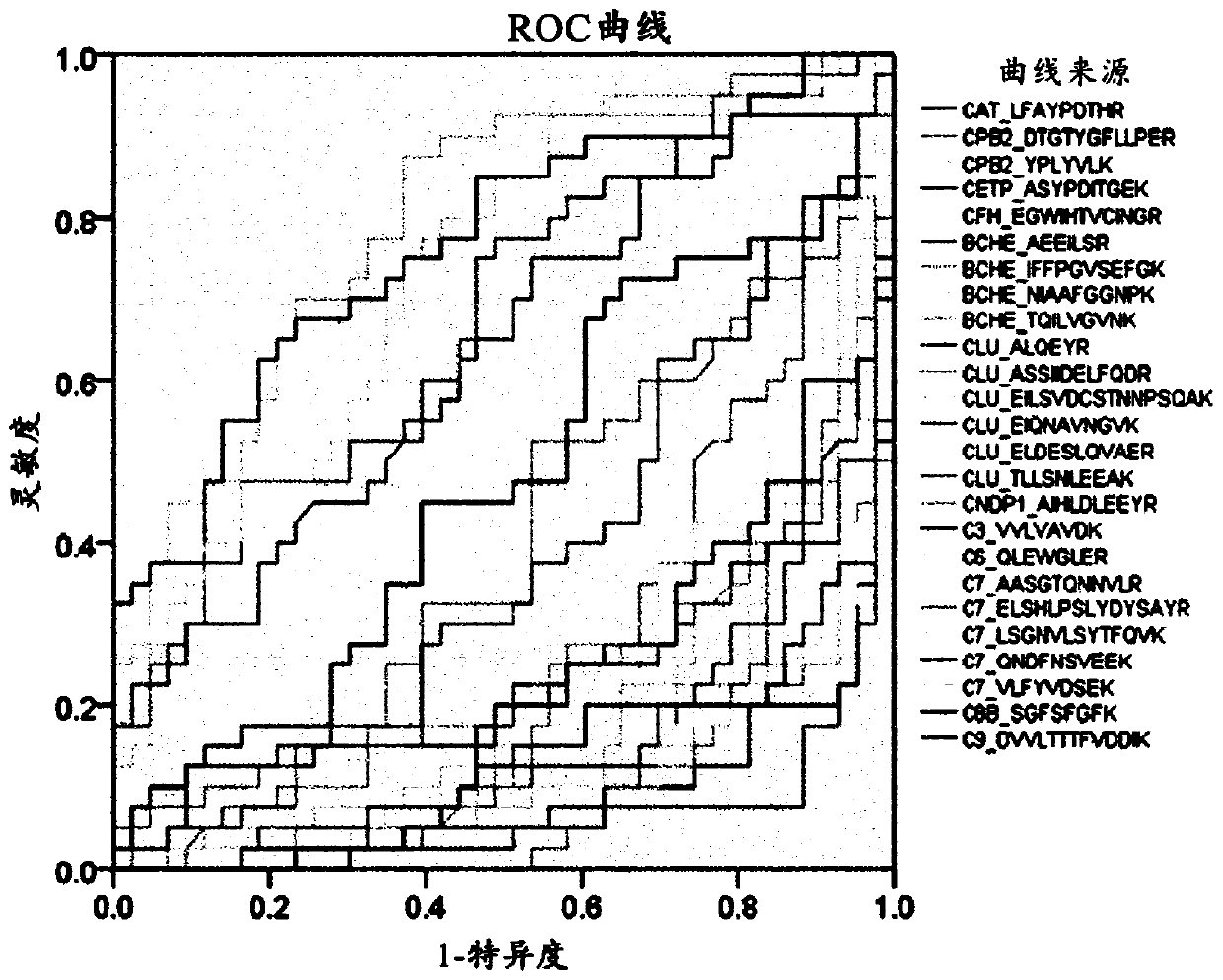
[0135] Example 2: Marker Screening
Embodiment 2-1
[0136] Example 2-1: Selection of marker target candidate groups
[0137] 1,152 proteins were screened from the Liver Atlas database (LiverAtlas database), the most comprehensive comprehensive resource related to liver diseases, and 59 proteins were screened from the Human Protein Atlas (Human Protein Atlas). Six pairs (nonnal=6, HCC= 6) Screening of 1,304 proteins in proteome profiling of tissue samples (tissue). Moreover, 28 proteins approved by the US Food and Drug Administration (FDA) or clinical laboratory self-construction project (Laboratory Developed Test, LDT) plus one liver cancer diagnostic marker developed in previous research , A total of 2189 proteins known to be associated with liver disease (Hepatic disease) were selected. In order to select only blood proteins secreted into blood that can be quantitatively analyzed in the 2189 proteins, a total of eight prediction tools are used, namely the plasma proteome database (Plasma proteome database), target P (TargetP...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com