Method for removing free chlorine from nickel chloride electrodeposition anolyte
A technology of anolyte and free chlorine, applied in chemical instruments and methods, water pollutants, water/sewage treatment, etc., can solve the problems of poor operating environment at the production site and poor dechlorination effect of anolyte, and achieve good dechlorination effect , low chlorine content, meet the effect of recycling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
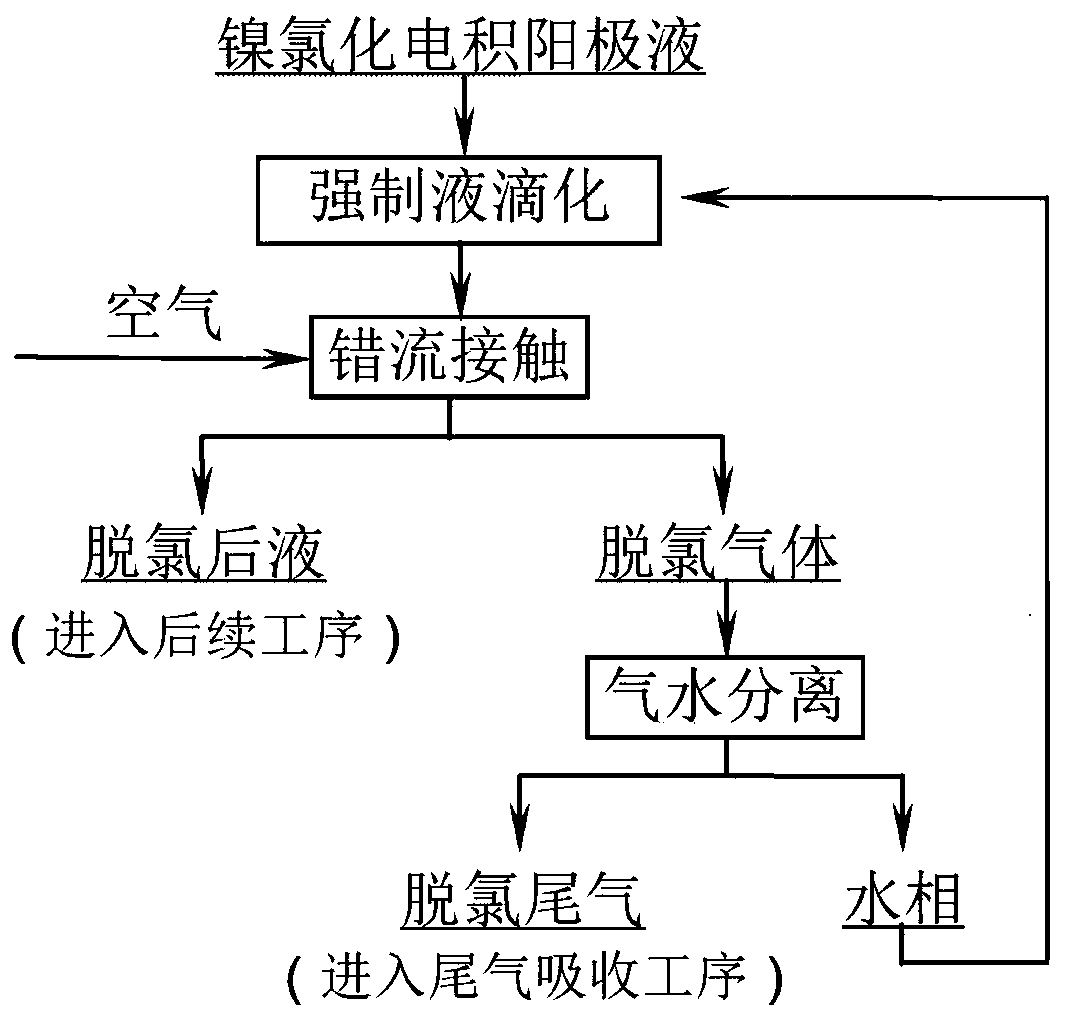
[0020] A method for removing free chlorine from a nickel chloride electrowinning anolyte, the specific implementation process: the temperature is 80°C, the concentration of free chlorine is 400ppm, the pH is 1.2, and the volume is 1m 3 The nickel chloride electrowinning anolyte is forced into droplets to form a droplet with a radius of 1mm, and the nickel chloride electrowinning anode droplet passes through 10m 3 The cross-flow of the air and the anolyte droplet are fully mixed and contacted, and the air carrying the chlorine gas escapes to the gas-water separation device, the separated water phase is combined with the nickel chloride electrowinning anolyte, and the dechlorinated liquid enters the subsequent process for analysis and detection The free chlorine concentration of the liquid after dechlorination is 6ppm, and the removal rate of free chlorine is 98.5% through calculation.
Embodiment 2
[0022] A method for removing free chlorine from a nickel chloride electrowinning anolyte, the specific implementation process: the temperature is 80°C, the concentration of free chlorine is 413ppm, the pH is 2.0, and the volume is 1.2m 3 The nickel chloride electrowinning anolyte is forced to droplet to form a droplet with a radius of 2mm, and the nickel chloride electrowinning anode droplet passes through 20m 3 The cross-flow of the air and the anolyte droplet are fully mixed and contacted, and the air carrying the chlorine gas escapes to the gas-water separation device, the separated water phase is combined with the nickel chloride electrowinning anolyte, and the dechlorinated liquid enters the subsequent process for analysis and detection The chlorine gas concentration of the liquid after dechlorination is 5.5ppm, and the removal rate of free chlorine is calculated to be 98.7%.
Embodiment 3
[0024] A method for removing free chlorine from a nickel chloride electrowinning anolyte, the specific implementation process: the temperature is 80°C, the concentration of free chlorine is 448ppm, the pH is 2.2, and the volume is 1.5m 3 The nickel chloride electrowinning anolyte is forced into droplets to form a droplet with a radius of 3mm, and the nickel chloride electrowinning anode droplet passes through 50m 3 The air cross-flow is fully mixed with the anolyte droplets, and the air carrying chlorine gas escapes to the gas-water separation device, the separated water phase is combined with the nickel chloride electrowinning anolyte, and the dechlorinated liquid enters the subsequent process, and the analysis and detection results The chlorine gas concentration of the liquid after dechlorination is 4.5ppm, and the removal rate of free chlorine is calculated to be 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com