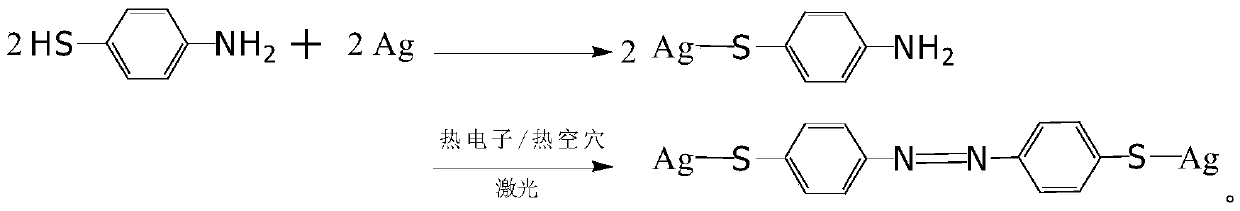
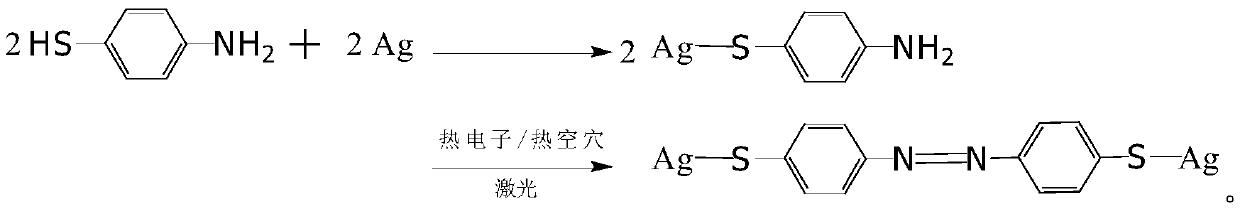
Method for preparing p-mercaptoazobenzene by using p-amino diphenyl disulfide as raw material
A technology of aminodiphenyl disulfide and mercaptoazobenzene, which is applied in thiol preparation, organic chemistry and other directions, can solve the problems of harsh reaction conditions, does not meet the requirements of green chemistry, potential toxicity, etc., and achieves a guaranteed yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Preparation of p-aminodiphenyl disulfide ethanol solution: because p-aminodiphenyl disulfide is insoluble in water, in order to reduce the pollution of organic solvents to the environment, absolute ethanol is used as solvent in this patent, and p-aminodiphenyl disulfide ether for dissolution. Measure an appropriate amount of p-aminodiphenyl disulfide and dissolve it in absolute ethanol, form a p-aminodiphenyl disulfide ethanol solution after completely dissolving, and set aside;
[0018] Coarsening of silver foil: When the laser is irradiated on the surface of precious metals such as gold and silver, due to the oscillation of surface plasmons, hot electron / hot hole pairs will be formed on the metal surface, and hot electron / hot hole pairs have a good The catalytic effect of p-aminodiphenyl disulfide has been proved by experiments to generate p-mercaptoazobenzene through hot electron / hot hole catalysis, and the hot electron / hot hole generated after the surface of silver ...
Embodiment 2
[0025] (1) Dissolve 2.5g p-aminodiphenyl disulfide in 10L absolute ethanol, fully dissolve;
[0026] (2) Take silver foil with a purity of 99.99%, and 2 SO 4 330~405g / L, CrO 3 Coarse treatment in 400-430g / L roughening solution for 5 seconds;
[0027] (3) Ultrasonic cleaning, hot water washing, cold water washing, and running water washing are performed on the roughened silver foil to clean the surface of the silver foil;
[0028] (4) Drying the cleaned silver foil naturally;
[0029] (5) Dip the dried silver foil into the ethanol solution of p-aminodiphenyl disulfide, and stir it mechanically for 5 minutes;
[0030] (6) Take out the silver foil and dry it naturally;
[0031] (7) Fully irradiate the silver foil with a laser with a wavelength of 532 nm to form p-mercaptoazobenzene on the surface of the silver foil.
Embodiment 3
[0033] (1) Take 0.025g p-aminodiphenyl disulfide and dissolve it in 10L absolute ethanol, fully dissolve;
[0034] (2) Take silver foil with a purity of 99.99%, and carry out roughening treatment in a roughening solution of 100-400 g / L nitric acid for 1-10 seconds;
[0035] (3) Wash the roughened silver foil with cold water and running water, and clean the surface of the silver foil;
[0036] (4) Drying the cleaned silver foil naturally;
[0037] (5) Dip the dried silver foil into the ethanol solution of p-aminodiphenyl disulfide, and stir it mechanically for 10 minutes;
[0038] (6) Take out the silver foil and dry it naturally;
[0039] (7) Fully irradiate the silver foil with a laser with a wavelength of 633nm to form p-mercaptoazobenzene on the surface of the silver foil.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap