Human leukocyte protease inhibitor and its recombinant preparation and application
A technology of protease inhibition and white blood cells, applied in the field of biomedicine, can solve the problems of lack of effective drugs, etc., and achieve the effect of inhibiting the weight gain of adipose tissue, inhibiting hepatic steatosis, and reducing body weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] A method for constructing, expressing and purifying recombinant human leukocyte protease inhibitor WAP, SWAP1, and SWAP2 proteins, the method comprising the following steps:
[0051] (1) Construction of recombinant plasmids pET-WAP, pET-SWAP1, pET-SWAP2 A1) According to the sequence published on the biological information website, design and synthesize primers to construct pet-WAP plasmid: pET-6*his-enterokinase site -WAP, the primer sequences are respectively SEQ ID NO:4 and SEQ ID NO:5; pET-SWAP1 plasmid: pet-6*his-enterokinase site-SWAP1, the primer sequences are respectively SEQ ID NO:6 and SEQ ID NO :7; pET-SWAP2 plasmid: pet-6*his-enterokinase site-SWAP2, the primer sequences are respectively SEQ ID NO:8 and SEQ ID NO:9; the role of the enterokinase site is in the process of protein refolding Promote the correct folding of proteins and increase the renaturation yield of proteins;
[0052] A2) Extract the DNA of human lung tissue and use it as a template, and use ...
Embodiment 2
[0068] WAP protein, SWAP1 protein and SWAP2 protein increased hWAP content in serum of mice.
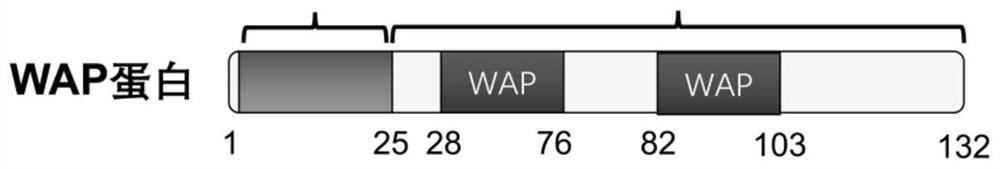
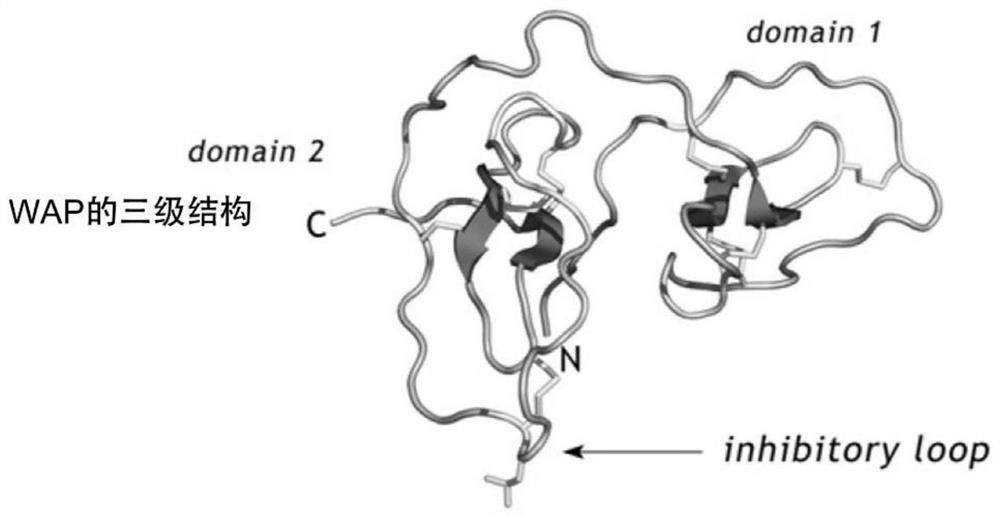
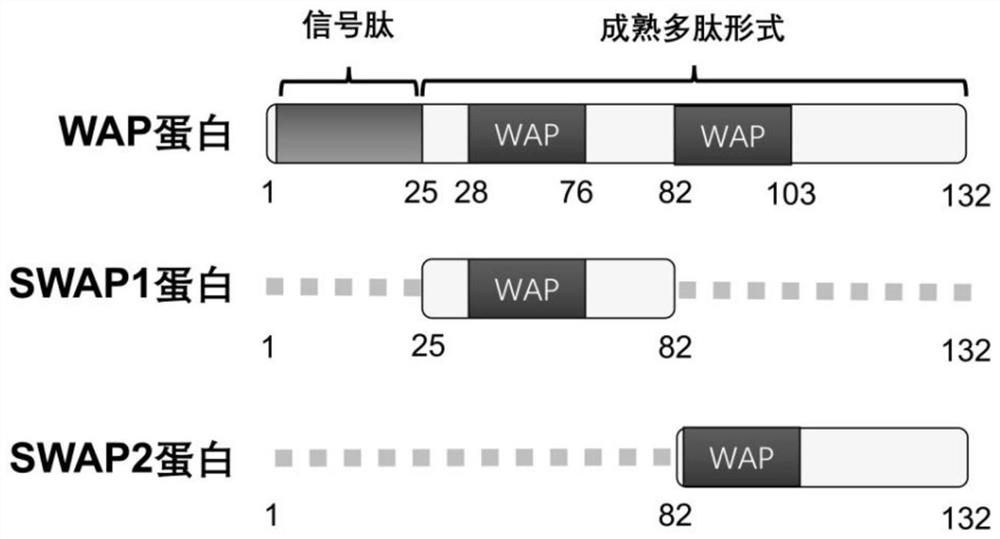
[0069] The C57BL / 6 mice used in the examples of the present invention were purchased from the Experimental Animal Center of Hubei Province. Feeding mice with a high-fat diet is a classic mouse model that mimics type 2 diabetes. Before WAP injection, 4-week-old C57BL / 6 mice were fed a high-fat diet for 14 weeks. The mice in the HFD group were randomly divided into 4 groups: the HFD group was injected with normal saline (Saline group), the HFD group was injected with WAP full-length protein (WAP group); the HFD group was injected with WAP short peptide 1 (SWAP1 group); the HFD group was injected with WAP short peptide 2 (SWAP2 group), the injected WAP protein, SWAP1 protein and SWAP2 protein sequences are as follows figure 2 shown. Formal injection of WAP and its two short peptides, subcutaneous injection, each injection at a dose of 0.2 μg / g body weight, once a day, for a total of...
Embodiment 3
[0072] WAP protein, SWAP1 protein and SWAP2 protein reduced the body weight of mice, but did not affect the food intake of mice.
[0073] After 5 days of injection of WAP protein, SWAP1 protein and SWAP2 protein, statistical calculation was performed on the body weight change (△BW) of the mice compared with before injection: compared with the HFD+Saline group, the WAP protein, SWAP1 protein and SWAP2 protein group The body weight of the mice decreased significantly ( Figure 6); Simultaneously, the food intake (Food intake) of each group of mice was counted, and there was no significant difference between the food intake of each group of mice ( Figure 7 ), indicating that the short-term injection of WAP protein, SWAP1 protein and SWAP2 protein can significantly reduce the body weight of C57BL / 6 mice fed with high-fat diet. At the same time, the injection of WAP protein, SWAP1 protein and SWAP2 protein does not affect the body weight of mice.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com