Antimalarial dimer immunoadhesin, pharmaceutical composition and application
A technology of immunoadhesin and dimer, which is applied in the direction of drug combination, antibody mimic/stent, anti-infective drug, etc., can solve the problems of unknown biological activity and anti-disease effect of immunoadhesin, and achieve in vivo stability Excellent, broad clinical application prospects, the effect of prevention and treatment of malaria
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1. Construction and expression of soluble dimeric immunoadhesin
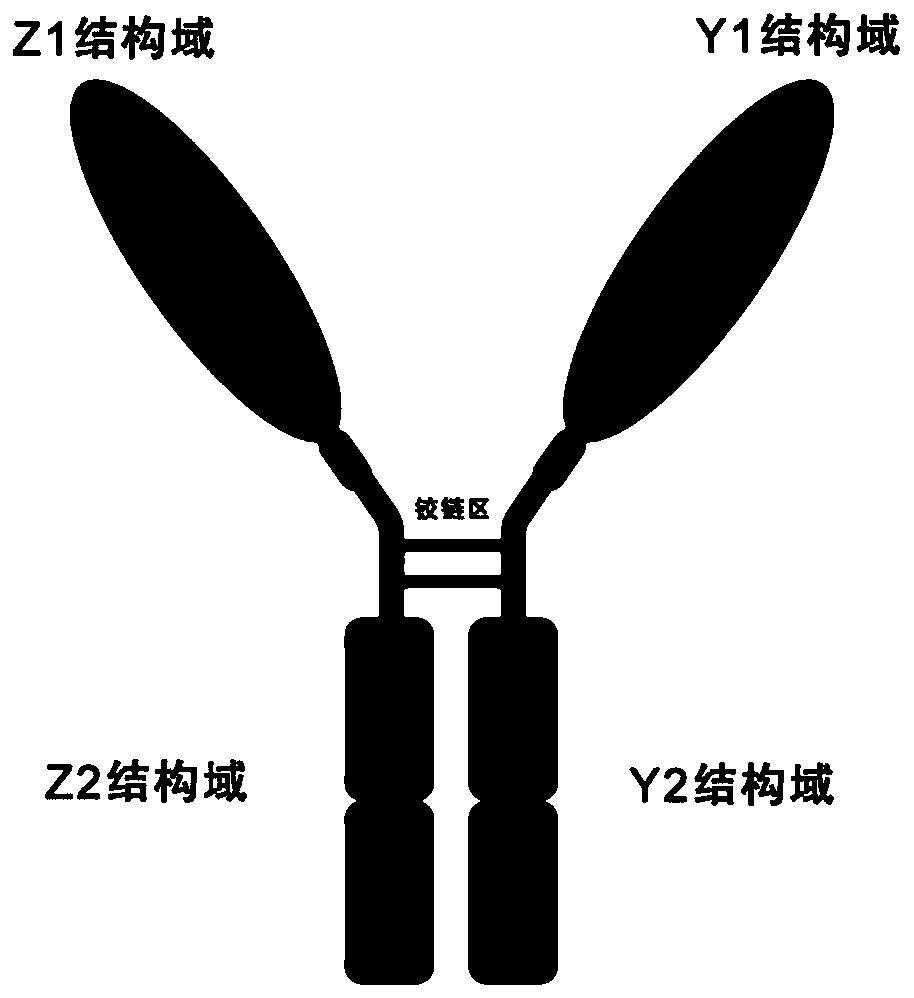
[0038] Such as figure 1 As shown, the soluble antimalarial dimer immunoadhesin is a dimer with antibody IgGFc, and the method of constructing and expressing the dimer immunoadhesin itself is a routine experimental technique in the field, and is briefly described as follows :
[0039] (1) Whole gene synthesis of soluble dimeric immunoadhesin BSG-Fc (comprising two polypeptide chains, the amino acid sequence and nucleotide sequence of each polypeptide chain are shown in SEQ ID NO.2 and SEQ ID NO.12) BSG / GYPA-Fc (comprising two polypeptide chains, the amino acid sequence and nucleotide sequence of the first polypeptide chain are shown in SEQ ID NO.4 and SEQ ID NO.13, the amino acid sequence of the second polypeptide chain and The nucleotide sequence is shown in SEQ ID NO.5 and SEQ ID NO.14); BSG / GYPB-Fc (comprising two polypeptide chains, the amino acid sequence and nucleotide sequence of the first...
Embodiment 2
[0042] Example 2. In vivo stability test of soluble dimeric immunoadhesin
[0043] The half-life of antimalarial dimeric immunoadhesin was evaluated in NOG mice using the literature (Hu S, et al. Science translational medicine, 2017, 9(380): eaag0339.) method. The results showed that the in vivo half-lives of BSG-Fc, BSG / GYPA-Fc, BSG / GYPB-Fc, and BSG / GYPC-Fc were 8.9 days, 8.1 days, 7.5 days, and 8.4 days, respectively; the positive control cetuximab was 8.6 days; while the half-lives of the three BSG antigen peptides (amino acid sequence TNINTLENSDHTCFAR; SNPYFIVGSR; ENYYNSDIAGPAR) were too short to be detected, and the half-life of the BSG extracellular protein half-life was too short to be detected.
Embodiment 3
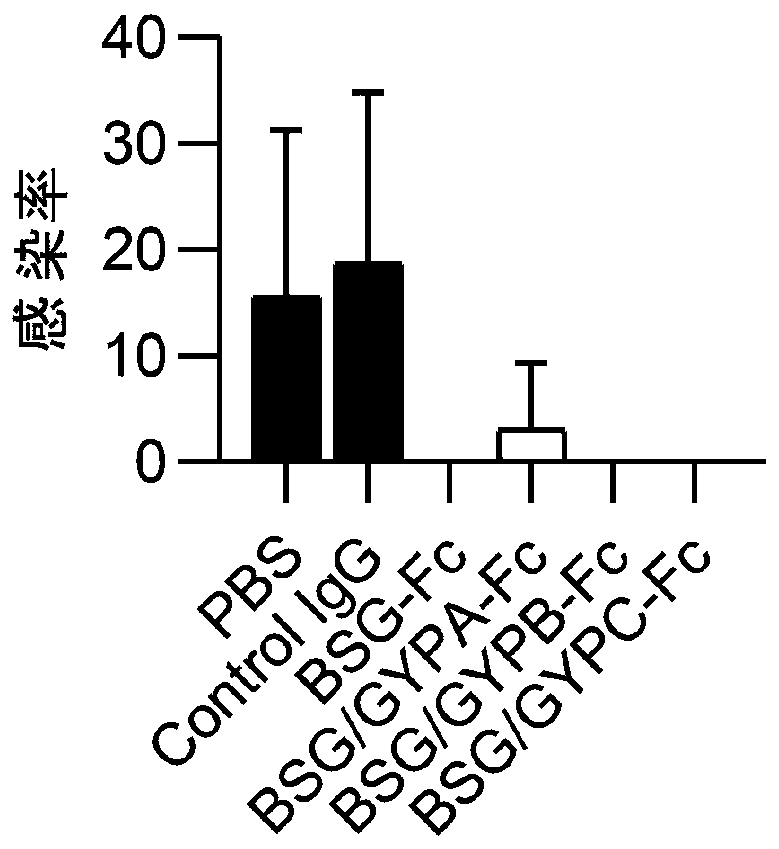
[0044] Example 3. In vitro invasion experiment of soluble dimeric immunoadhesin
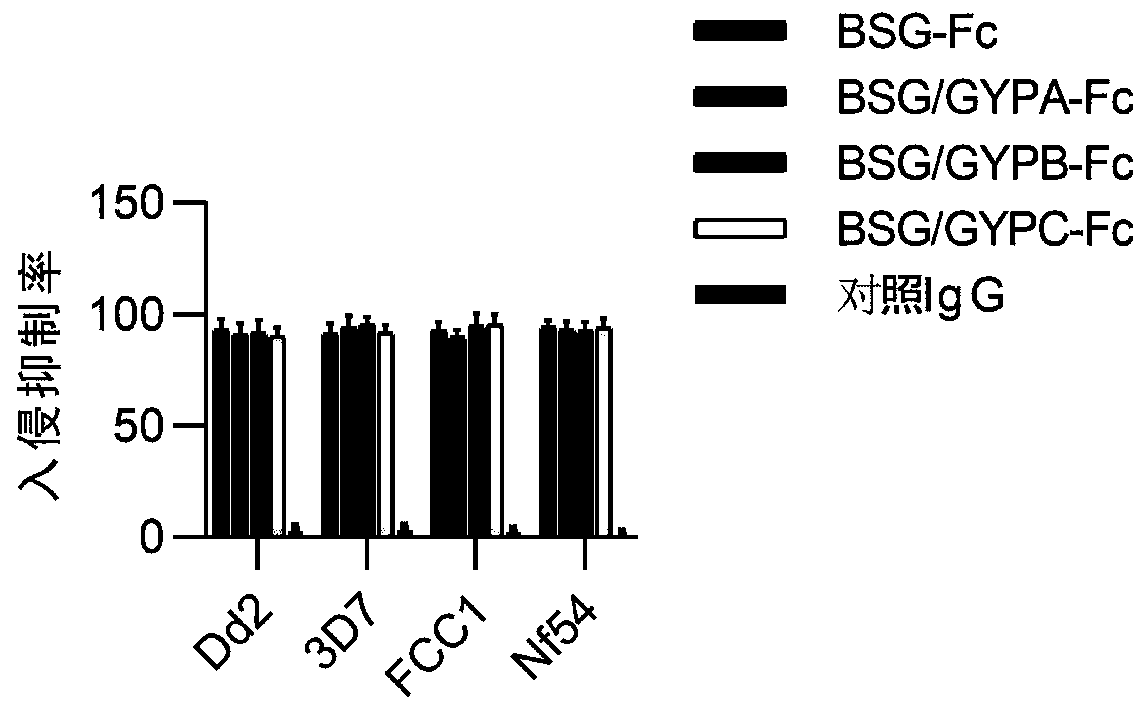
[0045] Type O human erythrocytes were used to conduct the Plasmodium invasion experiment, and the test method was the same as the literature (Zhang M Y, etal.blood, 2018, 131(10):1111-1121.). Four kinds of Plasmodium beads Dd2, 3D7, FCC1, and Nf54 were used to carry out this experiment, and healthy human IgG was used as the control group, and BSG-Fc, BSG / GYPA-Fc, BSG / GYPB-Fc, BSG / GYPC-Fc and control were administered respectively IgG, the dosage is 10 μg / ml. The calculation method of the invasion suppression rate is also the same as the literature, and the results are as follows figure 2 Shown: It shows that BSG-Fc, BSG / GYPA-Fc, BSG / GYPB-Fc, BSG / GYPC-Fc have a strong ability to inhibit invasion, and the invasion inhibition rate is close to 100%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com