Anti-PD-L1 antibody and application thereof
A PD-L1 and antibody technology, applied in the field of biomedicine, can solve the problems of large dosage, small range of indications, low response rate, etc., and achieve good in vivo stability and good ADCC activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0107] Example 1: Preparation of anti-human PD-L1 antibody hybridoma cells
[0108] Immunization: Balb / c mice were immunized with human PD-L1 / mFc recombinant protein, and the serum titer was detected by ELISA with a 96-well microplate plate coated with human PD-L1-His recombinant protein; the serum titer reached the fusion requirement Mice were used for the next step of cell fusion.
[0109] Cell fusion and hybridoma preparation: On the 67th day after the initial immunization, select the mouse whose titer reached the requirement, aseptically take the spleen of the mouse, prepare B lymphocyte suspension, and mix it with FO myeloma cells at a ratio of 5:1. The two cells were fused under the action of PEG4000. After the fused cells were resuspended in HAT medium, they were divided into 96-well cell culture plates. Set at 37°C, 5% CO 2 Cultured in an incubator.
Embodiment 2
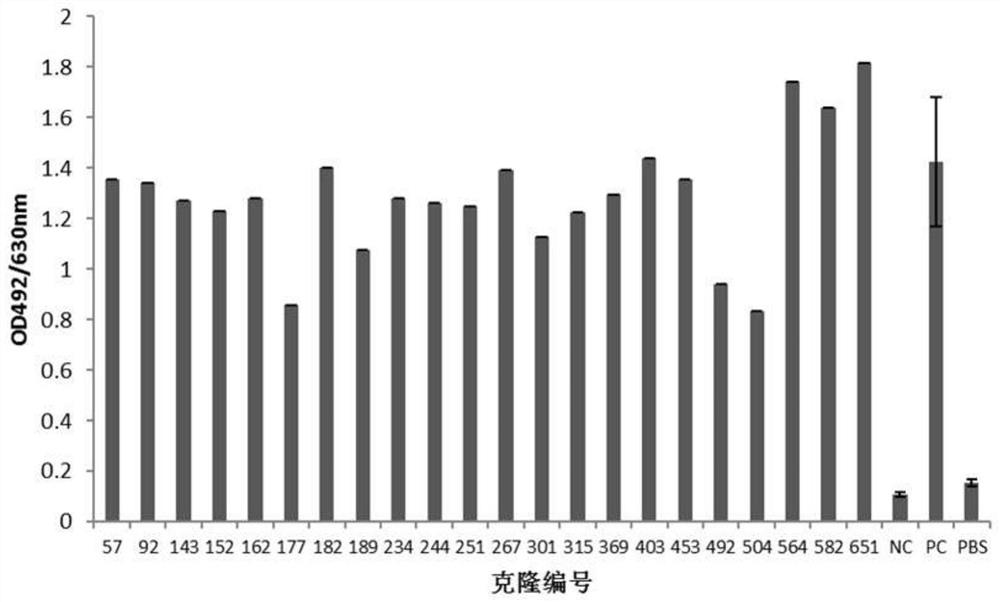
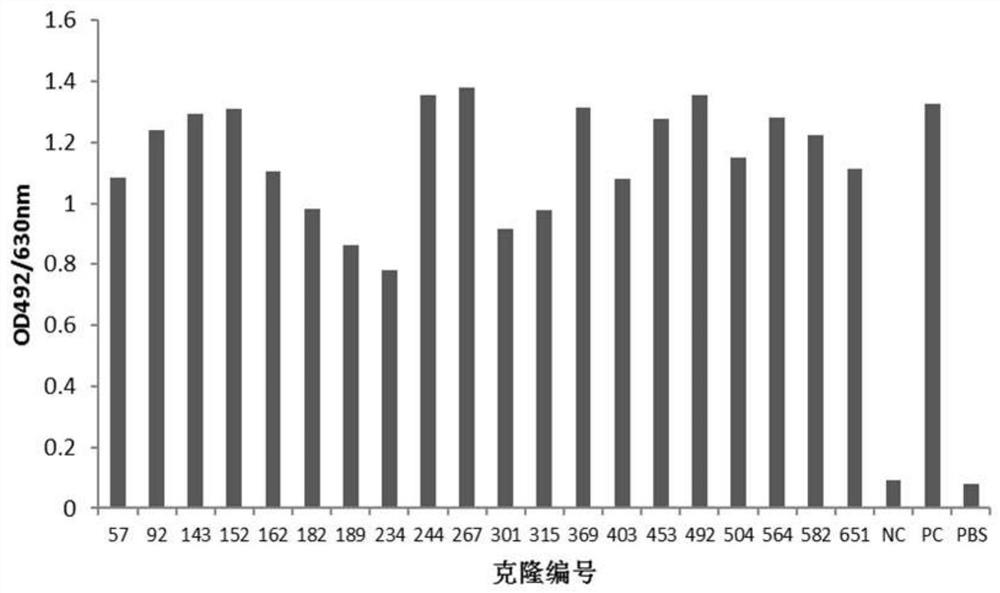
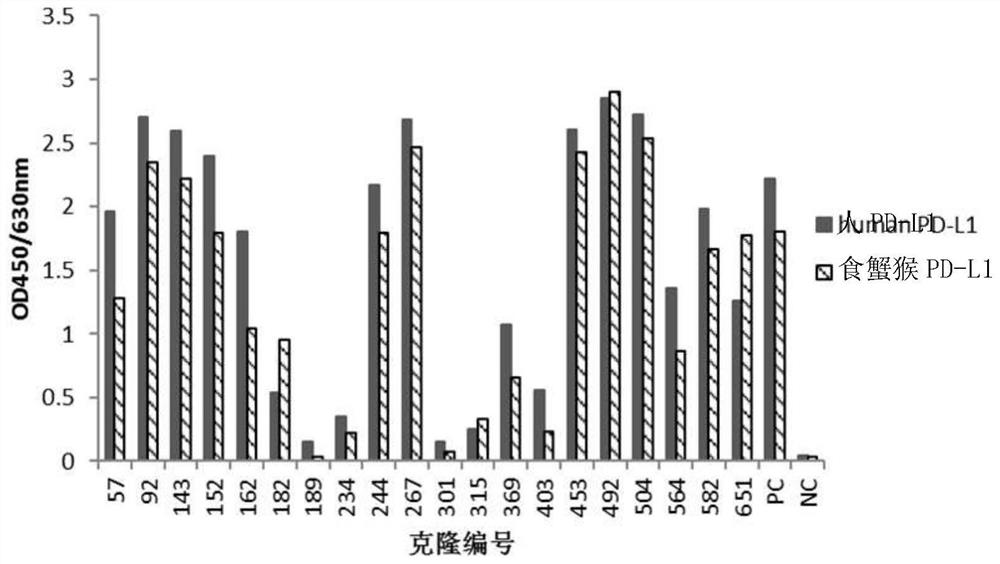
[0110] Example 2: Screening of anti-human PD-L1 antibody-positive hybridoma cell lines
[0111] 1. ELISA binding screening of positive hybridomas
[0112] 10-14 days after fusion, human PD-L1-His recombinant protein (10ug / ml, pH 9.6, 0.1M NaHCO 3 ) coated microtiter plate, 4°C, overnight; blocked with 4% skimmed milk powder-PBS, 37°C, 2h; washed three times with PBST (0.05% Tween20-PBS), added hybridoma clone culture supernatant, 37°C, 1h. Set up the following controls: (1) positive control (PC): immunized mouse serum (diluted with PBS 1:1000); (2) negative control (NC): pre-immunized mouse serum (diluted with PBS 1:1000); (3) Blank control: PBS. Washed three times with PBST (0.05% Tween20-PBS), added HRP-goat anti-mouse IgG (Fcγ), diluted 1:20000, 37°C, 1h; then washed five times with PBST (0.05% Tween20-PBS), added OPD color developing solution, avoid light for 10-15min, add 2MH 2 SO 4 Terminate the reaction; read the A492 value with a microplate reader. The A492 va...
Embodiment 3
[0122] Example 3: Sequence determination of murine anti-human PD-L1 antibody
[0123] After expanding the hybridoma cell No. 182 secreting anti-human PD-L1 antibody, use Mouse Monoclonal Antibody IgG Subclass Test Card (Cat: A12403, VicNovo) and Mouse Monoclonal Antibody Light / Heavy Chain Test Card (Cat: A12401, VicNovo) according to the reagents Subtype detection was carried out according to the operating procedures, and the subtype identification was as follows: the heavy chain was IgG1, and the light chain was Kappa chain.
[0124] Total RNA was extracted from the No. 182 hybridoma cells according to the instructions of the TRIzol kit (Cat: 15596026, Invitrogen); the total RNA of the hybridoma cells was reverse-transcribed into cDNA using M-MuLV reverse transcriptase (Cat: M0253S, NEB) ; Use degenerate primers (refer to the book [Dong Zhiwei, Wang Yan. Antibody Engineering (Second Edition). Beijing Medical University Press, 2001, 313-314]) and Phusion kit (Cat: E0553L, NE...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com