Methods for manufacturing adjuvant
A technology of body adjuvant and solvent, applied in the field of manufacturing adjuvant containing saponin, can solve the problem that molecular pathway is not clearly defined, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0451] Embodiment 1: the research of the first solution preparation method and composition
Embodiment 1A
[0452] Example 1A - Solvent Composition
[0453] method
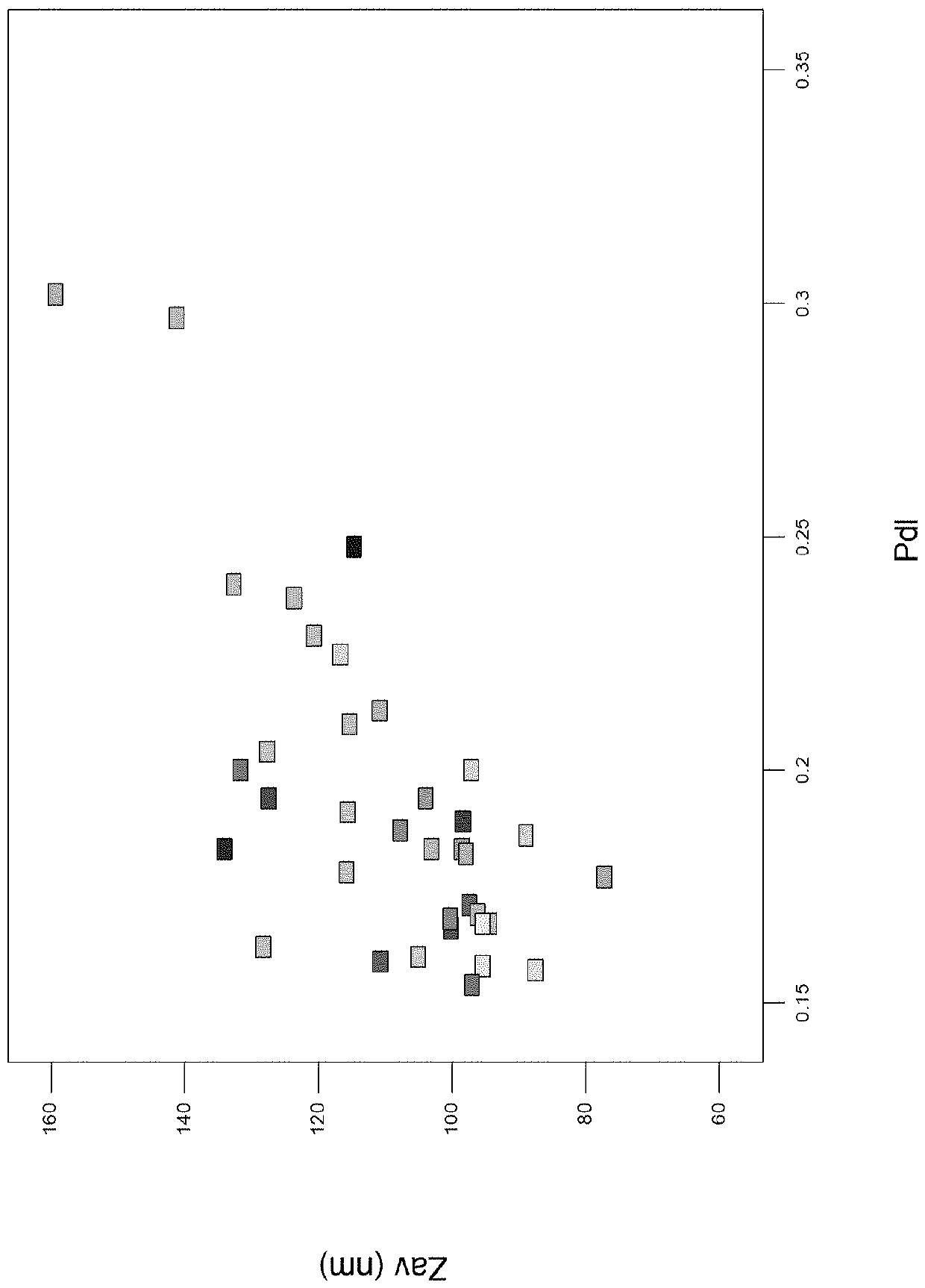
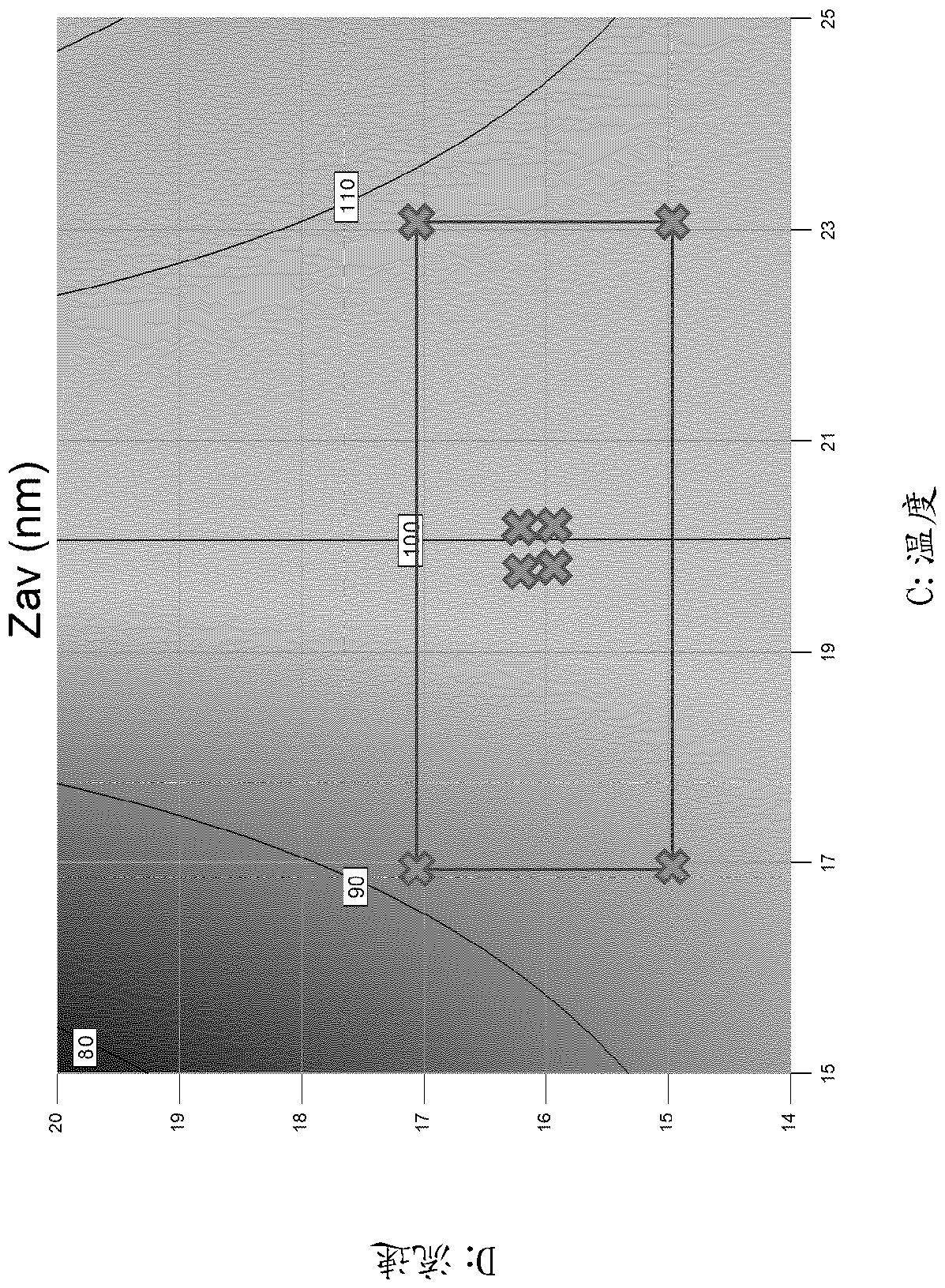
[0454] To study the effect of solvent composition on liposome production, solutions of DOPC, cholesterol and 3D-MPL were prepared at various ethanol / isopropanol ratios.
[0455] Each of DOPC, cholesterol and 3D-MPL was separately dissolved at 55° C. (60% by volume for DOPC, 20% for cholesterol, and 20% for 3D-MPL) for 15 minutes. The 3D-MPL solution was then added to the DOPC solution and this mixture was added to the cholesterol solution and mixed for a further 15 minutes to provide a final composition with 150 mg / ml DOPC (20:5:1 weight ratio DOPC :cholesterol:3D-MPL).
[0456] The single-chamber microfluidic device was operated at a total flow rate of 14 ml / min, a flow rate ratio of 20 (19:1) (1:19 organic phase:aqueous phase), using water for injection as the aqueous phase stock solution, and an environment at room temperature .
[0457] result
[0458] Table 1 – Effect of solvent composition on liposome...
Embodiment 1B
[0463] Example 1B - Solution Preparation
[0464] method
[0465] Evaluates the order in which components are added, which compares the two following methods:
[0466] 1. Separately dissolve DOPC, cholesterol and 3D-MPL (60%, 20%, 20% by volume) in 80:20 ethanol:IPA for 15 minutes at 55°C. The 3D-MPL solution was then added to the DOPC solution and further mixed for another 15 minutes. The 3D-MPL / DOPC mixture was then added to the cholesterol solution and mixed for a further 1 hour to provide a final composition with 120 mg / ml DOPC (20:5:1 weight ratio DOPC:cholesterol:3D-MPL).
[0467] 2. Suspend 3D-MPL with 50% solvent (80:20 ethanol:IPA), and then add to DOPC and cholesterol powder. The volume was then adjusted with the remainder of the solvent, and the mixture was heated to 40°C for 15 minutes to provide a final composition with 120 mg / ml DOPC (20:5:1 weight ratio DOPC:cholesterol:3D-MPL).
[0468] Method 1 requires keeping the mixture at 55°C for 1 hour for comp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| area | aaaaa | aaaaa |
| area | aaaaa | aaaaa |
| area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com