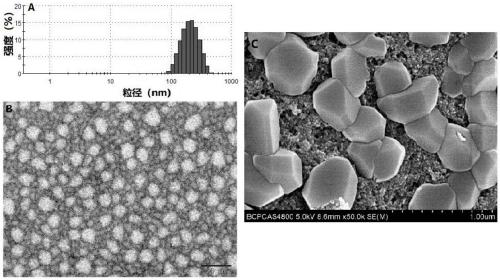
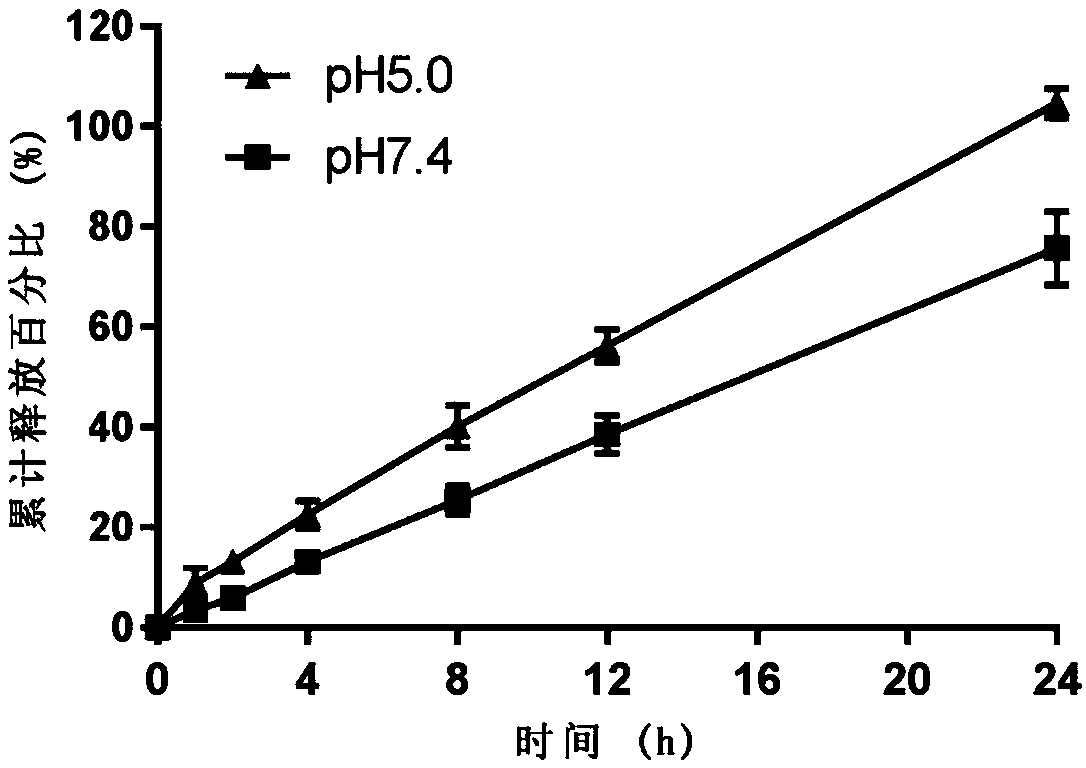
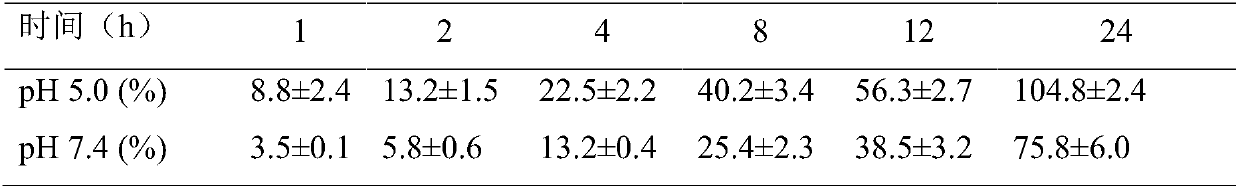
Small intestine transporter targeting nano calcium carbonate composition for improving oral absorption of insoluble drugs
A technology for insoluble drugs and calcium carbonate, which is applied in drug delivery, active ingredients of hydroxyl compounds, and pharmaceutical formulations, and can solve the problems of insufficient absorption and low efficiency of insoluble drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The preparation of embodiment 1 tablet
[0041] Add 45mL of a mixed solvent of ethanol and water (ethanol: water = 1:2, V / V) into a 200mL beaker, and add 1.0mL of 0.5M CaCl2 solution and 1.0mL of 100mg / mL polyacrylic acid dropwise at a speed of 0.5mL / min Sodium solution, stirred for 5min. Then add 1.0mL 0.5M Na dropwise at a rate of 0.5mL / min 2 CO 3 solution, stirred for 5min. Centrifuge at 8000rpm for 10min, and freeze-dry the precipitate to obtain calcium carbonate nanoparticle freeze-dried powder.
[0042] Take 100 mg of freeze-dried calcium carbonate nanoparticles, add 10 mL of 2 mg / mL paclitaxel methanol solution, add 10 mL of 1 mg / mL TPGS1000 methanol solution linked to Sar-Gly, ultrasonicate for 5 min, stir at room temperature for 20 min, and evaporate to dryness at 30 °C under reduced pressure solvent. Add 50mL HBSS buffer solution, ultrasonic 5min, stir at room temperature 10min, filter with 0.8 μm microporous membrane, obtain the calcium carbonate nanopar...
Embodiment 2
[0043] The preparation of embodiment 2 tablet
[0044] Dissolve 60mg of sodium alginate in deionized water at 30°C, then slowly add 7mL of 0.02M CaCl2 solution, and stir for 30min. Then 7 mL of 0.02M Na was added dropwise 2 CO 3 solution, stirred for 3h. Centrifuge at 8000rpm for 10min, wash several times, and freeze-dry the precipitate to obtain calcium carbonate nanoparticle freeze-dried powder.
[0045] Take 10 mg of freeze-dried calcium carbonate nanoparticles, add 1 mL of 0.5 mg / mL doxorubicin aqueous solution, add 1 mL of 1 mg / mL carnitine-linked Pluronic F68 aqueous solution, sonicate for 5 min, stir at room temperature for 10 h, centrifuge, and wash , and lyophilized to obtain a carnitine-modified calcium carbonate nanoparticle composition loaded with doxorubicin. Then pass the freeze-dried powder through a 80-mesh sieve, mix with microcrystalline cellulose, add starch slurry to make a soft material, granulate with a 14-mesh sieve, dry at 40°C, pass through a 12-me...
Embodiment 3
[0046] The preparation of embodiment 3 hard capsules
[0047] Add 10mL of 20mM Na to a 100mL beaker 2 CO 3 solution and polyethylene sulfonic acid, stirred for 1 min. Then slowly add 10mL of 20mM CaCl2 solution, react at room temperature for 10h. After centrifuging at 8000 rpm for 10 min, the precipitate was washed three times with Tris buffer solution of pH 9, and then freeze-dried to obtain freeze-dried powder of calcium carbonate nanoparticles.
[0048] Take 10 mg of freeze-dried calcium carbonate nanoparticles, add 2 mL of 1 mg / mL cyclosporine A methanol solution, add 1 mL of 1 mg / mL methanol solution of PEG2000-PLA1800 linked to deoxycholic acid, ultrasonicate for 5 min, and stir at room temperature for 0.5 h, the solvent was evaporated to dryness under reduced pressure at 30°C. Add 5mL HBSS buffer solution, ultrasonic 5min, stir at room temperature 10min, filter with 0.8 μm microporous membrane, obtain the calcium carbonate nanoparticle composition loaded with cyclos...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com