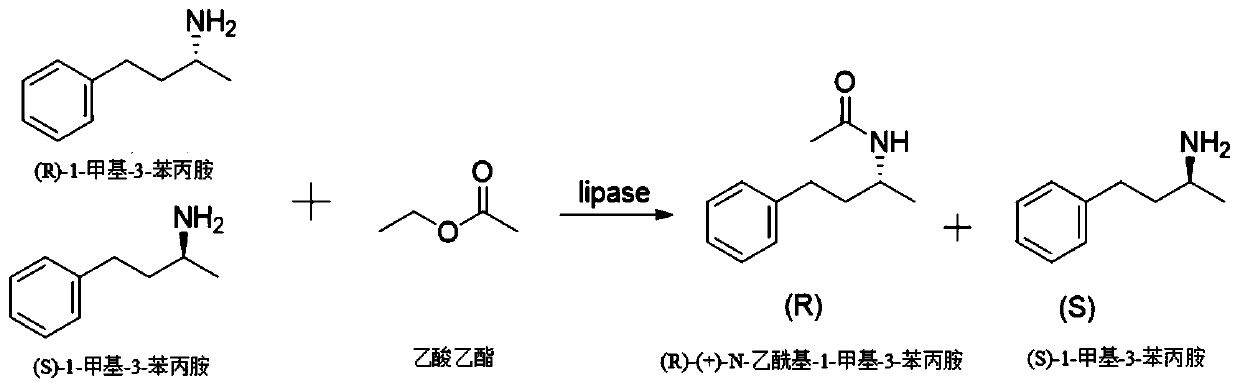
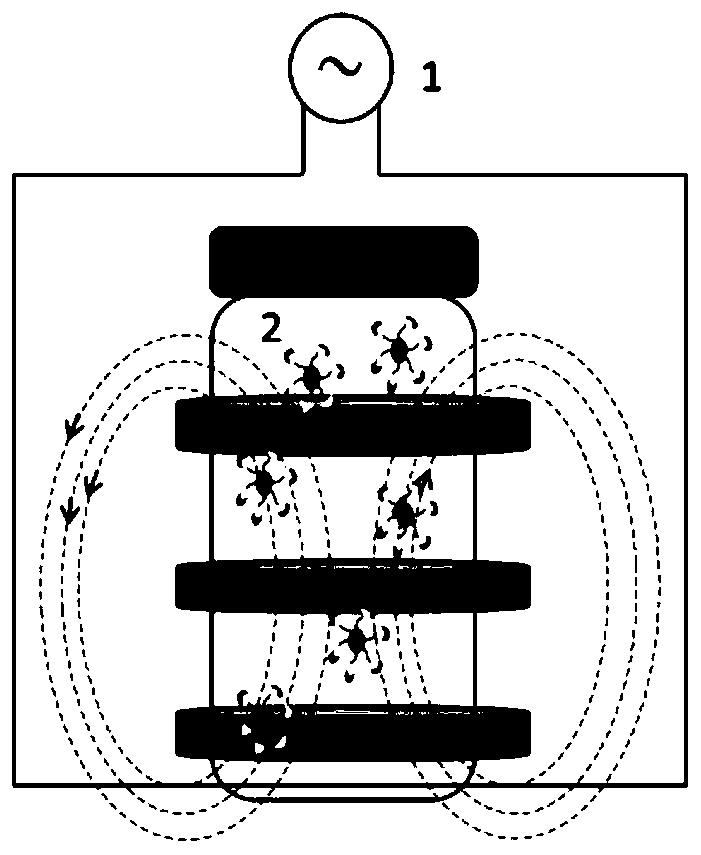
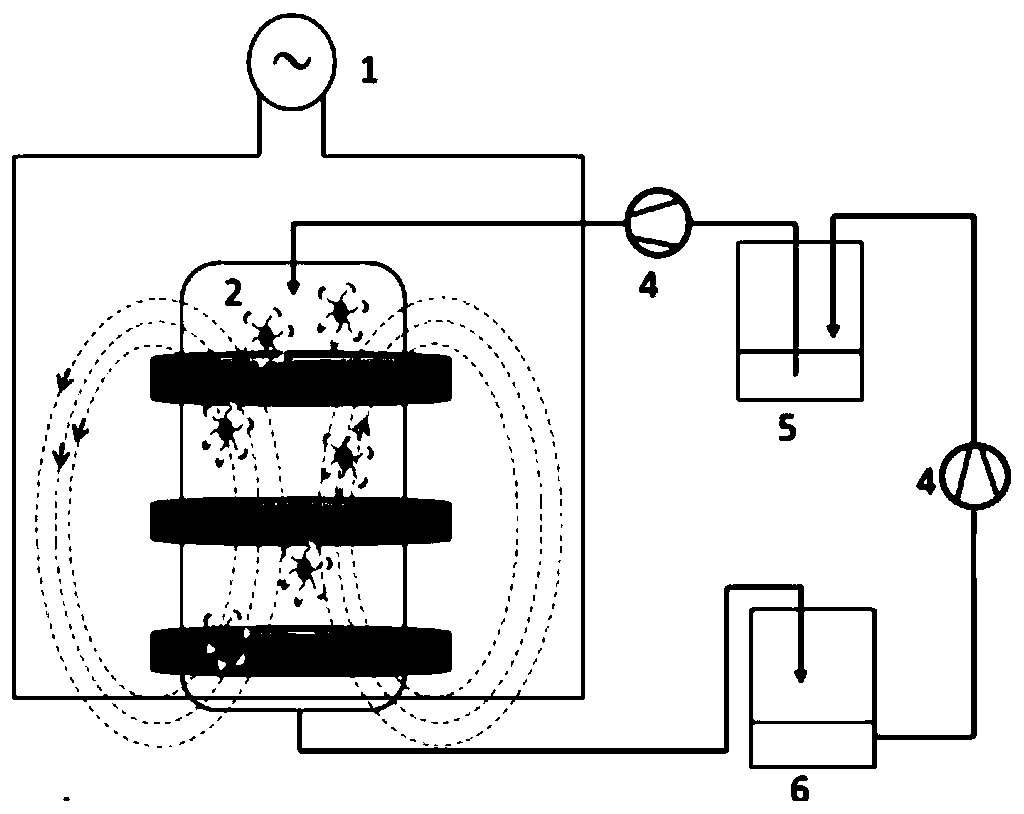
Magnetic immobilized lipase and application thereof in resolution of 1-methyl-3-amphetamine
A technology of immobilizing lipase and lipase, applied in the direction of immobilized lipase, lipase, enzyme, hydrolase, etc. on or in inorganic carrier, can solve the problems of reduced conversion efficiency, poor water solubility of substrate, restriction enzyme application, etc. High, splitting ability, the effect of optimizing immobilization conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1: Screening of free lipase
[0034] Take 100 mg of 12 kinds of lipases from different manufacturers (numbered for convenience to distinguish different manufacturers) into 10 mL reaction bottles of the same specification, respectively add solvent anhydrous toluene 1800 μL, acylating reagent ethyl acetate 200 μL, substrate 60 μL of 1-methyl-3-amphetamine was reacted in a shaker at 40°C and 150 rpm for 5 days. After the reaction, the enzyme was centrifuged from the reaction solution, and the organic solvent was removed by a rotary evaporator to obtain a transparent crystal. Add 2 mL of iso Dissolve propanol, filter with an organic filter head with a pore size of 0.45 μm, and analyze (S)-1-methyl-3-amphetamine and (R)-(+)-N-acetyl-1-methyl- 3-amphetamine content, determination of substrate conversion and enantiomeric excess of (R)-(+)-N-acetyl-1-methyl-3-amphetamine. The results are shown in Table 1. The results show that the lipase LIP10 has the best enantiose...
Embodiment 2
[0037] Example 2: Preparation of Magnetically Immobilized Lipase
[0038] (1) Fe 3 o 4 Magnetic nanoparticles: 5.6g FeSO 4 ·7H 2 O and 10 g FeCl 3 ·6H 2 O was respectively dissolved in 40mL distilled water, mixed and put into a 250mL three-neck flask, 150mL deionized water was added, stirred at a high speed at 1000r / min and 40°C, and 40mL ammonia water (NH 3 ·H 2 O solution), when the solution becomes black and bright and the pH of the supernatant is ≥ 10.0, adjust the temperature to 60°C and continue stirring for 30min, then adjust the temperature to 70°C and stir for 1h. After the reaction is over, use absolute ethanol and Repeated washing with deionized water 10 times until the supernatant was neutral (i.e. pH 7.0), recovered by a magnet, frozen in an ultra-low temperature -80°C refrigerator for 7 hours, and then freeze-dried at -60°C for 12 hours to obtain Fe 3 o 4 Magnetic nanoparticles 2.8g, particle size 12-30nm.
[0039] (2) APTES-Fe 3 o 4 Magnetic nanoparti...
Embodiment 3
[0042] Embodiment 3: the influence of reaction time on the selective acylation of 1-methyl-3-amphetamine
[0043]In six 10ml reaction flasks, add 1800 μL of solvent anhydrous toluene, 200 μL of acylating reagent ethyl acetate, 60 μL of substrate 1-methyl-3-amphetamine, and 100 mg of magnetically immobilized lipase prepared by the method in Example 2, respectively. 1d, 2d, 3d, 4d, 5d, 6d were respectively reacted in a shaker at 40°C and 150rpm. After the reaction, use an external magnetic field to recover the precipitate, separate the enzyme from the reaction solution, and use a rotary evaporator to remove the organic solvent to obtain a transparent crystal, add 2 mL of isopropanol, filter with an organic filter head with a pore size of 0.45 μm, and analyze by HPLC The content of (S)-1-methyl-3-amphetamine and (R)-(+)-N-acetyl-1-methyl-3-amphetamine in the sample determines the substrate conversion rate and (R)-( +)-N-acetyl-1-methyl-3-amphetamine enantiomeric excess, the resu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap