Mitoxantrone-containing medicine, and preparation method, pharmaceutical composition and application thereof
A technology of mitoxantrone and drugs, applied in the field of drugs containing mitoxantrone, can solve the problems of impact, toxicity and transfection activity, self-design contradictions, difficult connection and non-toxic degradation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0157] According to the second aspect of the present application, there is also provided a method for preparing the above-mentioned mitoxantrone-containing drug, which includes the following steps: providing any one of the above-mentioned nucleic acid nanoparticles; by means of physical connection and / or covalent connection The mitoxantrone is mounted on the nucleic acid nanoparticles to obtain the mitoxantrone-containing medicine.
[0158] When physically linked, mitoxantrone usually physically intercalates between GC base pairs. In the case of covalent linkage, mitoxantrone usually undergoes a chemical reaction with the exoamino group of the G ring to form a covalent linkage. The mitoxantrone-containing drug prepared by the above method can have better targeting after the target head is modified, can deliver mitoxantrone stably, and has high reliability.
[0159] In a preferred embodiment, the step of mounting mitoxantrone by physical connection includes: mixing and stirrin...
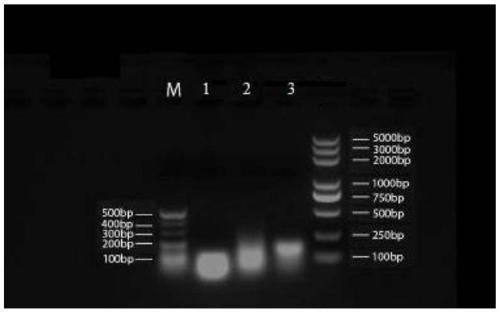
Embodiment 1
[0182] 1. RNA and DNA nanoparticle carriers:
[0183] (1) The three polynucleotide base sequences that make up the RNA nanoparticles, see Table 1 for details:
[0184] Table 1:
[0185]
[0186] (2) Three polynucleotide base sequences of DNA nanoparticles.
[0187] The DNA uses the same sequence as the above RNA, except that T is substituted for U. Among them, the molecular weight of chain a is 8802.66, the molecular weight of chain b is 8280.33, and the molecular weight of chain c is 9605.2.
[0188] The a, b, and c strands of the above-mentioned RNA nanoparticles and DNA nanoparticles were all synthesized by Sangon Bioengineering (Shanghai) Co., Ltd.
[0189] 2. Self-assembly experimental steps:
[0190] (1) RNA or DNA single strands a, b, and c are simultaneously mixed and dissolved in DEPC water or TMS buffer at a molar ratio of 1:1:1;
[0191] (2) Heat the mixed solution to 80°C / 95°C (the RNA assembly temperature is 80°C, and the DNA assembly temperature is 95°C),...
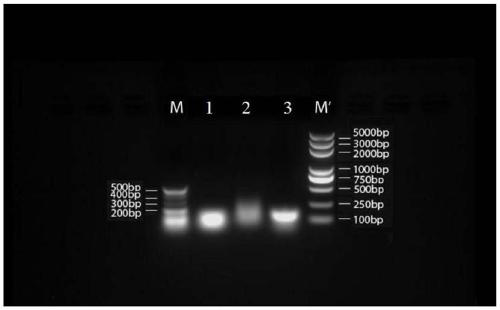
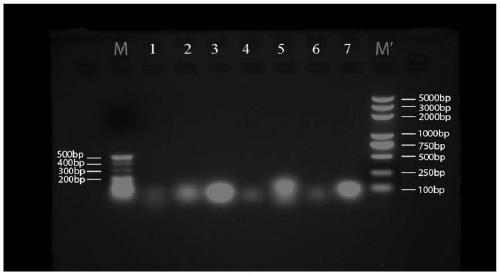
Embodiment 2
[0202] 1. Seven groups of short-sequence RNA nanoparticle carriers:
[0203] (1) Seven groups of three polynucleotide base sequences that make up RNA nanoparticles:
[0204] Table 2: R-1:
[0205]
[0206]
[0207] Table 3: R-2:
[0208]
[0209] Table 4: R-3:
[0210]
[0211] Table 5: R-4:
[0212]
[0213]
[0214] Table 6: R-5:
[0215]
[0216] Table 7: R-6:
[0217]
[0218] Table 8: R-7:
[0219]
[0220]
[0221] The single strands of the above seven groups of short-sequence RNA nanoparticle carriers were all synthesized by Sangon Bioengineering (Shanghai) Co., Ltd.
[0222] 2. Self-assembly experimental steps:
[0223] (1) RNA single strands a, b, and c are simultaneously mixed and dissolved in DEPC water or TMS buffer at a molar ratio of 1:1:1;
[0224] (2) Heat the mixed solution to 80°C, keep it for 5min and then cool down slowly to room temperature at a rate of 2°C / min;
[0225] (3) Load the product onto an 8% (m / v) non-denat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com