Medicine containing methotrexate, its preparation method, pharmaceutical composition and application thereof
A methotrexate and drug technology, applied in the field of medicine, can solve the problems of limited clinical application of the delivery reliability of small molecule drug methotrexate, contradiction between toxicity and transfection activity in its own design, difficult connection and non-toxic degradation, etc. Achieve high reliability, improve stability, and reduce the chance of contact
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
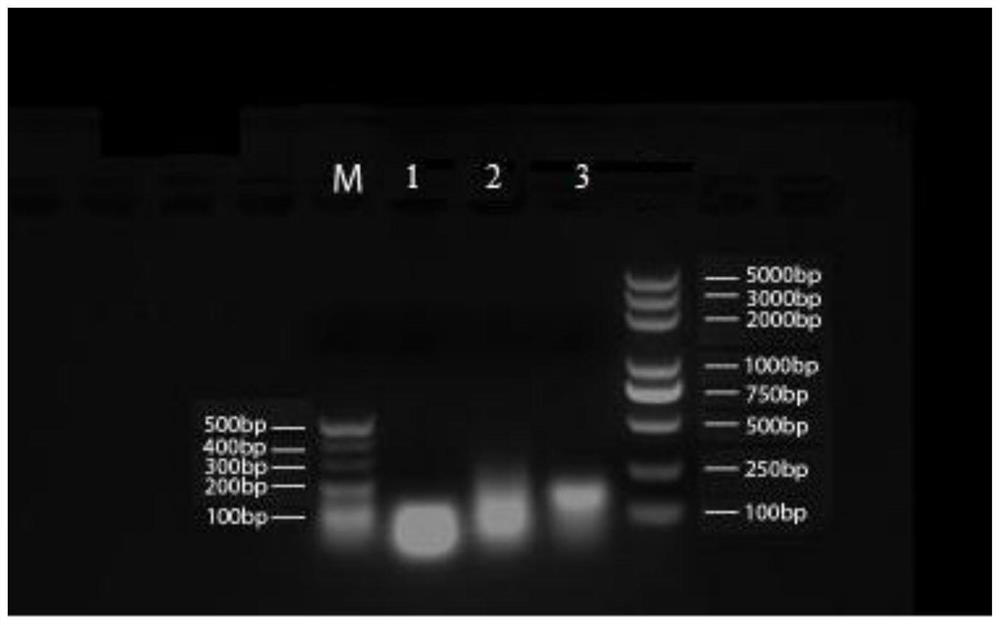
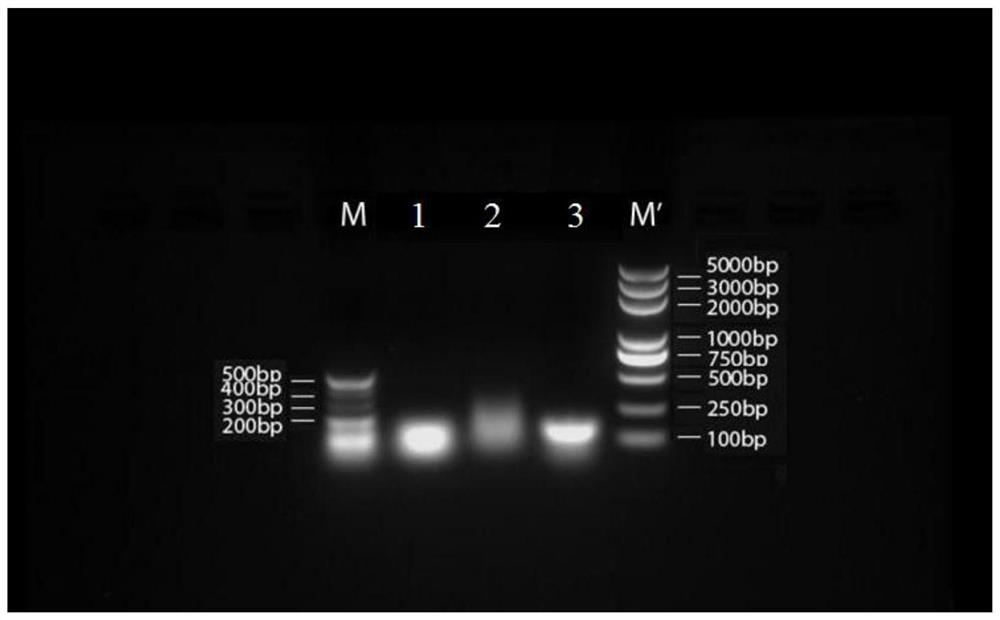
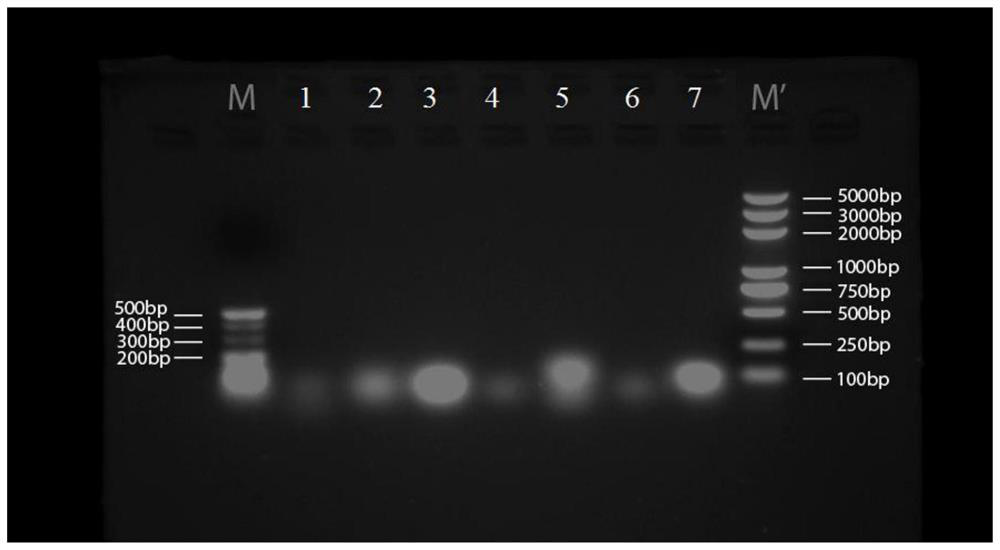
Image
Examples
preparation example Construction
[0156] According to the second aspect of the present application, there is also provided a method for preparing the above-mentioned methotrexate-containing drug, which includes the following steps: providing any one of the above-mentioned nucleic acid nanoparticles; by means of physical connection and / or covalent connection The methotrexate is mounted on the nucleic acid nanoparticles to obtain the methotrexate-containing medicine.
[0157] When physically linked, methotrexate is usually physically intercalated between GC base pairs. In the case of covalent linkage, methotrexate usually undergoes a chemical reaction with the amino group outside the G ring to form a covalent linkage. The methotrexate-containing drug prepared by the above method can have better targeting after the target head is modified, can stably deliver methotrexate, and has high reliability.
[0158]In a preferred embodiment, the step of mounting methotrexate through physical connection includes: mixing an...
Embodiment 1
[0182] 1. RNA and DNA nanoparticle carriers:
[0183] (1) The base sequences of the three polynucleotides that make up the RNA nanoparticles are shown in Table 1 below:
[0184] Table 1:
[0185]
[0186] (2) Three polynucleotide base sequences of DNA nanoparticles
[0187] The DNA uses the same sequence as the above RNA, except that T is substituted for U. Among them, the molecular weight of chain a is 8802.66, the molecular weight of chain b is 8280.33, and the molecular weight of chain c is 9605.2.
[0188]The a, b, and c strands of the above-mentioned RNA nanoparticles and DNA nanoparticles were all synthesized by Sangon Bioengineering (Shanghai) Co., Ltd.
[0189] 2. Self-assembly experimental steps:
[0190] (1) RNA or DNA single strands a, b, and c are simultaneously mixed and dissolved in DEPC water or TMS buffer at a molar ratio of 1:1:1;
[0191] (2) Heat the mixed solution to 80°C / 95°C (the RNA assembly temperature is 80°C, and the DNA assembly temperature i...
Embodiment 2
[0202] 1. Seven groups of short-sequence RNA nanoparticle carriers:
[0203] (1) The base sequences of the three polynucleotides of the seven groups of RNA nanoparticles are shown in Table 2 to Table 8 below:
[0204] Table 2: Three polynucleotide base sequences of R-1
[0205]
[0206]
[0207] Table 3: Three polynucleotide base sequences of R-2
[0208]
[0209] Table 4: Three polynucleotide base sequences of R-3
[0210]
[0211] Table 5: Three polynucleotide base sequences of R-4
[0212]
[0213]
[0214] Table 6: Three polynucleotide base sequences of R-5
[0215]
[0216] Table 7: Three polynucleotide base sequences of R-6
[0217]
[0218] Table 8: Three polynucleotide base sequences of R-7
[0219]
[0220]
[0221] The single strands of the above seven groups of short-sequence RNA nanoparticle carriers were all synthesized by Sangon Bioengineering (Shanghai) Co., Ltd.
[0222] 2. Self-assembly experimental steps:
[0223] (1) RNA ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com