Active protein or polypeptides derived from human placenta and application of active protein or polypeptides derived from human placenta
A protein and human placenta technology, applied in the field of biomedicine, can solve the problems of no research on active ingredients, and further research on the biological effects of specific proteins and peptides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
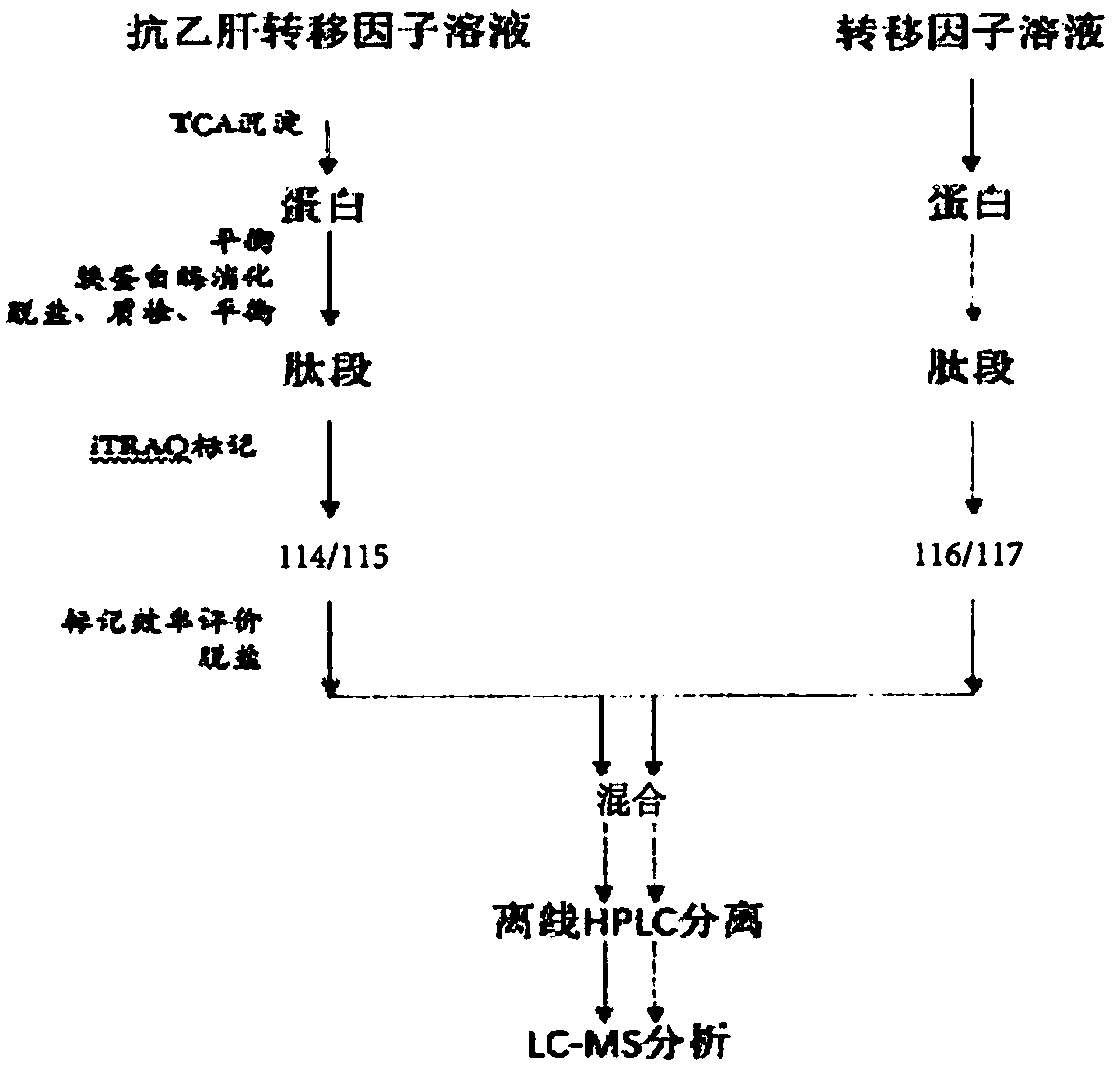
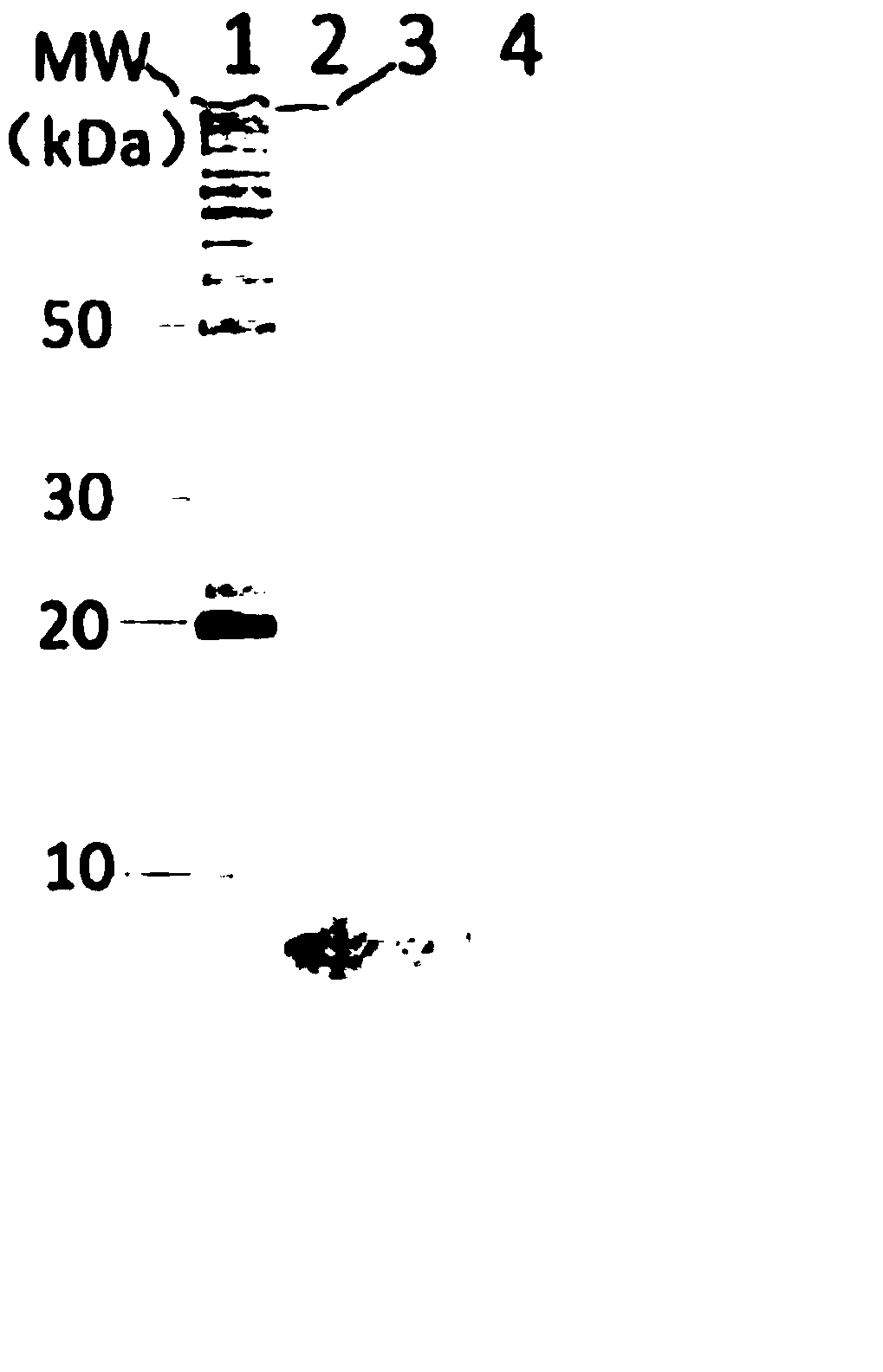
[0093] Example 1. Preparation of anti-hepatitis B placental transfer factor and placental transfer factor
[0094] (1) Pretreatment of human placenta material
[0095] For Anti-HBV Placental Transfer Factor (PSTF) :
[0096] Maternal physical examination standards: maternal serological test, hepatitis B virus surface antibody (HBsAb) positive; hepatitis B virus antigen and antibody HBsAg, HBeAg, HBeAb, HBcAb, hepatitis C virus (HCV), HIV (HIV), Patients with negative results for venereal pathogens (syphilis, gonorrhea, etc.).
[0097] Select the placenta that is positive for hepatitis B virus surface antibody (HBsAb), and negative for hepatitis B virus antigen and antibody (HBsAg, HBeAg, HBeAb, HBcAb), hepatitis C virus (HCV), HIV (HIV), and sexually transmitted disease pathogens . Those who suffer from malignant tumors, immunodeficiency diseases, acute infectious diseases, induced abortions, stillbirths, children with various deformities, abnormal appearance by naked eye...
Embodiment 2
[0115] Embodiment 2. placental transfer factor (PTF) and the immune activity test of anti-hepatitis B placental transfer factor (PSTF)
[0116] 1. Leukocyte Suspension Preparation
[0117] 20ml of peripheral blood from normal people, white blood cells were routinely separated, and the number of white blood cells was adjusted to 5×10 with Hank’s solution 6 pcs / ml, spare.
[0118] 2. HBVM positive serum
[0119] Detect the peripheral blood of HBsAg-positive patients whose HBsAg titer is above 1:512, put it in a sterile test tube, separate the serum routinely, and keep it for later use.
[0120] 3. Experimental method
[0121] 1) PSTF group
[0122] Take 0.5 ml of leukocyte suspension, add 0.5 ml of PSTF injection prepared in Example 1 (protein content greater than 0.5 mg / ml, ribose content greater than 80 μg / ml), and incubate at 37° C. for 30 min to make it sensitized. Remove the supernatant by centrifugation and adjust the number of cells to 5×10 6 pieces / ml.
[0123] Ta...
Embodiment 3
[0134] Example 3. Effect of placental transfer factor (PTF) and anti-hepatitis B placental transfer factor (PSTF) on mouse immune function
[0135] 1. Experimental animals
[0136] Kunming mice weighing between 18-22 g, 30 healthy pups, male and female, were randomly divided into 3 groups.
[0137] 2. Experimental method
[0138] With a frequency of once a day, the mice were injected intraperitoneally with the liquid of placental transfer factor (PTF) and anti-hepatitis B placental transfer factor (PSTF) prepared in Example 1 respectively, and the dose was 0.1ml / kg, carried out 7 times in total ; Use saline as a control.
[0139] On the 8th day of the experiment, the mice were plucked from the eyeballs to get blood, 0.02ml was added to 0.38ml white blood cell diluent, and the number of white blood cells was calculated after mixing; while staining (using Wright's staining method) to test the classification of white blood cells. Then the thymus and spleen of the mice were rem...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap