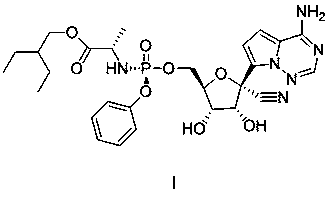
Preparation method of remdesivir
A compound and organic base technology, applied in the field of preparation of remdesivir, can solve the problems of increasing the risk of genotoxic impurities and achieve the effect of reducing the risk of genotoxic impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Preparation of Compound IV:
[0027] Under the protection of nitrogen, add compound V (16.48g, 47.4mmol) and pentafluorophenol (8.28g, 45.0mmol) into the round bottom flask, cool down to 0°C, then slowly add triethylamine (6.85mL) dropwise Dichloromethane solution (30mL), keep the temperature of the solution in the round bottom flask not higher than 5°C, after the dropwise addition, slowly return to room temperature (15~20°C), and stir at room temperature for 24h; add cold water (ice + water 100g in total, cool down the system to 0~5°C, pay attention to add ice to cool down first, but do not have solid ice, other examples are the same), after stirring, separate layers, wash the organic layer with saturated saline, and wash the organic layer with 10gMg 2 SO 4 Stir dry. After filtration, the organic layer was concentrated, then 100 mL of toluene was added, and evaporated to dryness under reduced pressure to obtain 21.18 g of crude compound IV. The crude product was dir...
Embodiment 2
[0035] Preparation of Compound IV:
[0036] Under the protection of nitrogen, add compound V (18.95g, 54.5mmol) and pentafluorophenol (8.28g, 45.0mmol) into the round bottom flask, cool down to 0°C, then slowly add triethylamine (8.18mL) dropwise Dichloromethane solution (30mL), keep the temperature of the solution in the round bottom flask not higher than 10°C, after the dropwise addition, slowly return to room temperature (15~20°C), and stir at room temperature for 12h; add cold water (ice + water (total 100g, the system was cooled to 0~5°C), after stirring, the layers were separated, the organic layer was washed with saturated brine, and the organic layer was washed with 10gMg 2 SO 4 Stir dry. After filtration, the organic layer was concentrated, then 100 mL of toluene was added, and evaporated to dryness under reduced pressure to obtain 21.62 g of crude compound IV. The crude product was directly used for the next step of resolution without further purification, and the ...
Embodiment 3
[0043] Preparation of Compound IV:
[0044] Under nitrogen protection, compound V (22.71g, 65.3mmol) and pentafluorophenol (8.28g, 45.0mmol) were added to a round bottom flask, cooled to 0°C, and then slowly added dropwise with triethylamine (7.68mL) Dichloromethane solution (30mL), keep the temperature of the solution in the round bottom flask not higher than 5°C, after the dropwise addition, slowly return to room temperature (15~20°C), and stir at room temperature for 16h; add cold water (ice + water (total 100g, the system was cooled to 0~5°C), after stirring, the layers were separated, the organic layer was washed with saturated brine, and the organic layer was washed with 10gMg 2 SO 4 Stir dry. After filtration, the organic layer was concentrated, then 100 mL of toluene was added, and evaporated to dryness under reduced pressure to obtain 20.95 g of crude compound IV. The crude product was directly used for the next step of resolution without further purification, and t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com