A pharmaceutical aerosol composition
A technology of composition and aerosol applied in the field of pharmaceutical aerosol composition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
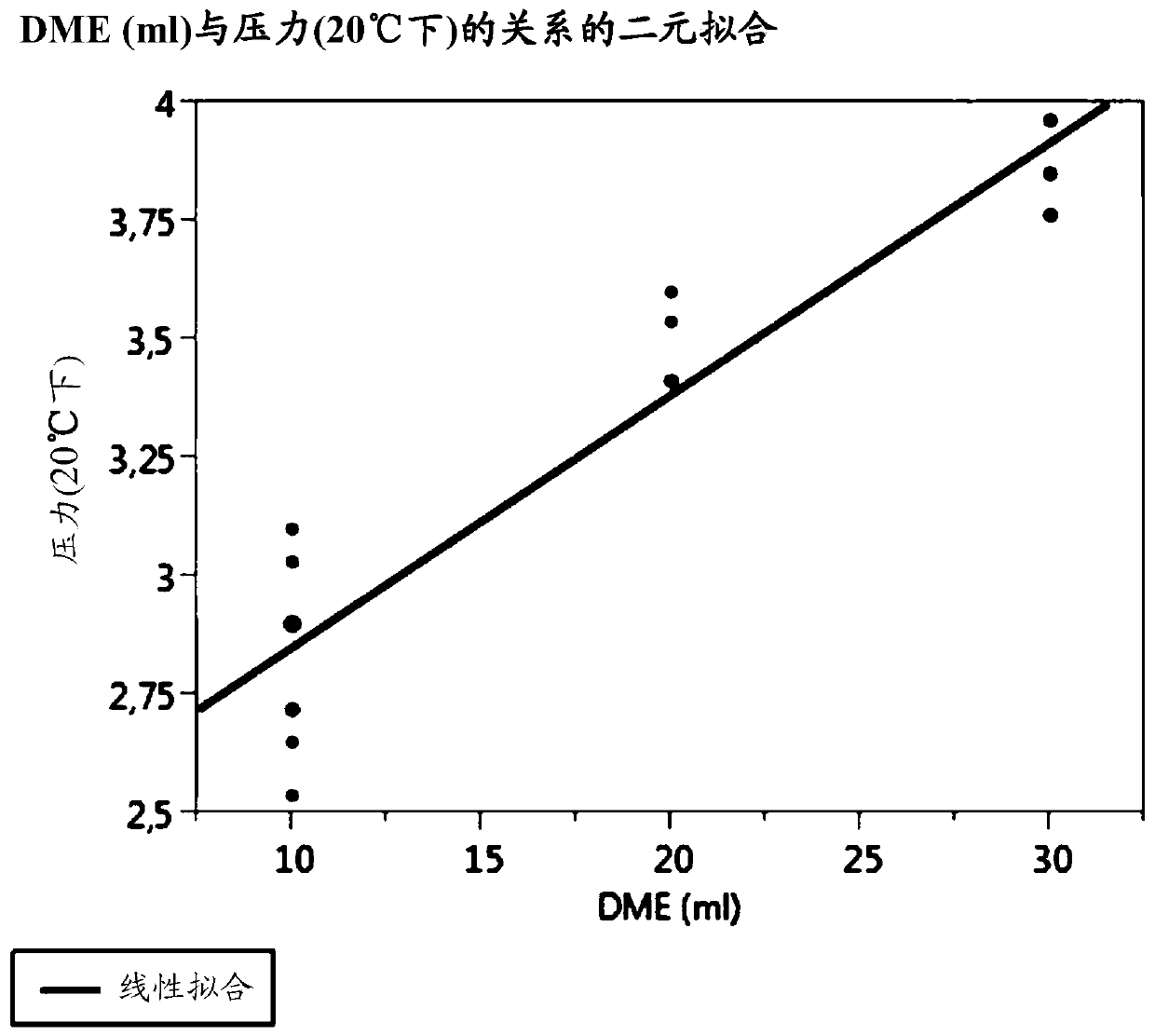
[0047] During the research leading up to the present invention, it was found that when dimethyl ether was used as a propellant, tacrolimus could be dissolved in the composition without adding a co-solvent while maintaining chemical stability. However, if Figure 1A As shown in , there is a linear relationship between the amount of dimethyl ether included in the composition and the vapor pressure inside the aerosol container, and ultimately the number of droplets with a size of 10 μm or less. For example, it has been found that when the amount of dimethyl ether in a composition further comprising 60 ml butane and 30 g of a hydrophobic carrier is 10 ml or the amount of dimethyl ether in a composition further comprising 50 ml of butane and 30 g of a hydrophobic carrier When it is 20ml, on the one hand, tacrolimus can be completely dissolved in the composition, and on the other hand, the spray rate of the composition discharged from the container can be adjusted so as to avoid form...
Embodiment 1
[0089] Compositions of the invention
[0090] Composition A
[0091]
[0092] Composition B
[0093]
[0094] Composition C
[0095]
[0096] Composition D
[0097]
[0098] Composition E
[0099]
[0100] Composition F
[0101]
[0102] Composition G
[0103]
[0104] Composition H
[0105]
[0106] Composition I
[0107]
[0108] Composition J
[0109]
[0110] Composition K
[0111]
[0112] Composition L (60:10)
[0113] components quantity Tacrolimus(*) 0.42mg (0.042%w / w) Edetate 0.042mg (0.0042%w / w) White Vaseline a
419.53 mg (41.95% w / w) Dimethyl ether 93.66mg (9.37w / w%) n-butane 486.35mg (48.65w / w%)
[0114] Composition M: (55:15)
[0115] components quantity Tacrolimus(*) 0.42mg (0.042%w / w) White Vaseline a
417mg (41.7%w / w) Dimethyl ether 139.6mg (13.96% w / w) n-butane 443 mg (44.3% w / w)
[0116] (*) Tacrolimus was added as t...
Embodiment 2
[0126] The chemical stability of tacrolimus in compositions A to I was tested and the following results were obtained.
[0127] Composition A
[0128]
[0129] Composition B
[0130]
[0131] Composition C
[0132]
[0133] Composition D
[0134]
[0135] Composition E
[0136]
[0137] Composition F
[0138]
[0139] Composition G
[0140]
[0141] Composition H
[0142]
[0143] Composition I
[0144]
[0145]
[0146] Composition J
[0147]
[0148] Composition K
[0149]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting range | aaaaa | aaaaa |
| Melting range | aaaaa | aaaaa |
| Melting range | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com